Scientists Achieve the Impossible by Manipulating Molecules with Light
Harnessing Light to Manipulate Molecules
A research team led by Prof. Alberto Credi at the University of Bologna has achieved a groundbreaking scientific milestone by leveraging light-driven (photochemical) reactions and molecular self-assembly. The team successfully inserted a thread-like molecule into the cavity of a ring-shaped molecule, creating a high-energy configuration previously thought impossible under thermodynamic equilibrium. In essence, light has been harnessed to produce molecular structures beyond the limits of nature’s equilibrium-driven processes.

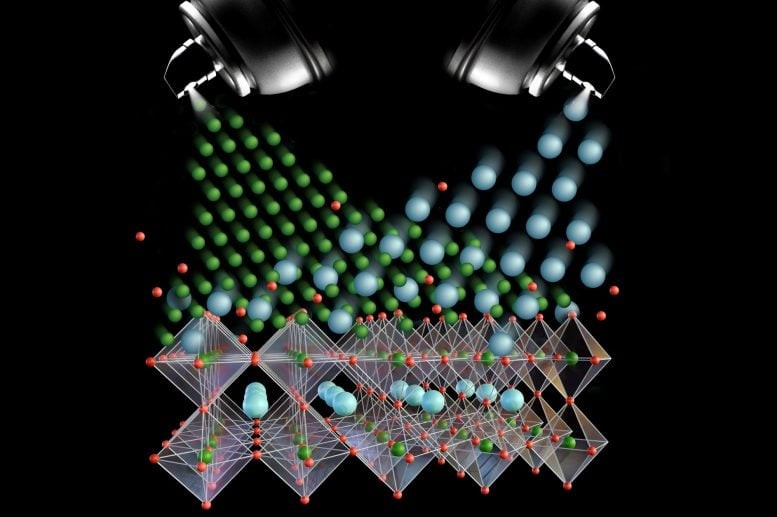
Figure 1. Scientists Accomplish the Impossible by Controlling Molecules with Light.
“We demonstrated that by applying light energy to an aqueous solution, we can prevent a molecular self-assembly reaction from reaching its thermodynamic minimum, resulting in a unique product distribution that cannot be achieved at equilibrium,” explains Alberto Credi. “This behavior, fundamental to many biological functions, is rarely explored in artificial molecules due to its complexity. Our simple and versatile approach, which relies on visible light – a clean and sustainable energy source – opens exciting opportunities for advancements in technology and medicine.” Figure 1 Shows Scientists Accomplish the Impossible by Controlling Molecules with Light.
Molecular Self-Assembly: A Foundation of Nanotechnology
Molecular self-assembly, a process where components naturally organize into nanometer-scale structures (1 nanometer = 1 billionth of a meter), is a cornerstone of nanotechnology. This phenomenon exploits molecules’ tendency to seek thermodynamic equilibrium – a state of minimum energy.
In contrast, living organisms rely on chemical transformations occurring far from equilibrium, driven by external energy inputs. Reproducing such mechanisms artificially poses a significant challenge but holds immense potential. Success in this domain could lead to groundbreaking innovations, including responsive materials, environmental sensors, and smart drugs designed to interact dynamically with biological systems.
Light-Induced Control of Molecular Orientation
In this study, the filament-like compound features two distinct ends, and the two rims of the cyclodextrin molecule also differ. When the former is inserted into the latter, two unique complexes are formed, differing in the relative orientation of the two components (as shown in the figure above).
Complex A is more stable than complex B, but complex B forms more rapidly. In the absence of light, only the thermodynamically favored complex, A, is observed at equilibrium. However, when the solution is exposed to visible light, the azobenzene molecule undergoes a structural change from an extended form (which fits within the cyclodextrin cavity) to a bent form, which is incompatible with the cavity. This transition causes the complex to dissociate.
Remarkably, light can also reverse this process, converting the azobenzene back from its bent form to the extended form, enabling the dissociated components to reassemble. Since complex B forms much faster than complex A, continuous illumination results in a steady state where complex B predominates. When the light is turned off, the azobenzene gradually reverts to its extended form, and after some time, complex A is the only form observed.
Dynamic Systems Beyond Thermodynamic Equilibrium
This self-assembly process, coupled with a light-driven chemical reaction, allows the use of light to accumulate unstable products. This innovation opens the door to new approaches in chemical synthesis, as well as the development of dynamic molecular materials and devices (such as nanomotors) that function under non-equilibrium conditions, much like the systems found in living organisms.
Source:SciTECHDaily
Cite this article:
Priyadharshini S (2024), Scientists Achieve the Impossible by Manipulating Molecules with Light, AnaTechMaz, pp. 87