A Reaction Inspired by Rocket Technology Produces Carbon with An Unprecedented Surface Area
Researchers have applied principles from rocket science to create carbon with an unprecedented surface area. This material can absorb approximately twice the amount of CO2 compared to current activated carbon options and demonstrates remarkable energy-storage potential.
As you may recall from high school experiments, like combining baking soda and vinegar to create a volcanic eruption, certain chemical reactions can lead to explosive results.
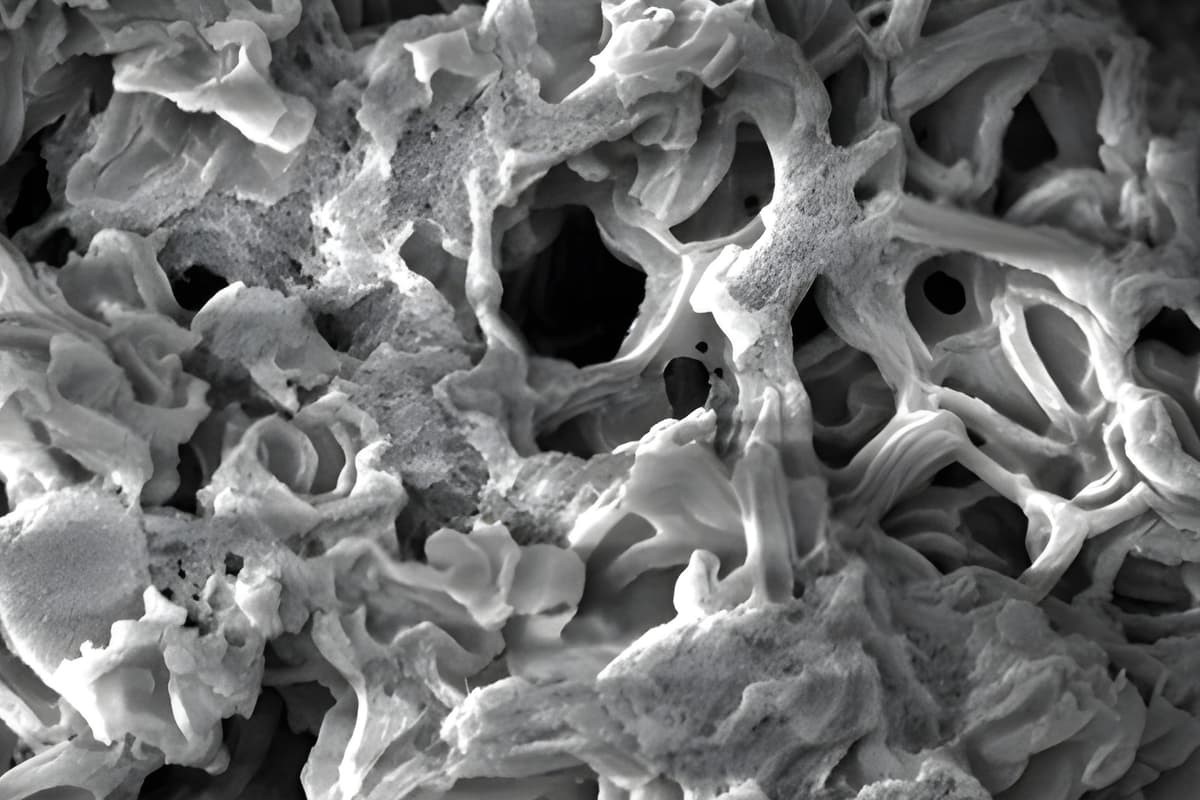
Figure 1. A close-up view of the new carbon material, which boasts the highest surface area ever recorded
Building on this principle in more serious applications, rocket scientists have been harnessing hypergolic reactions as fuel for various spacecraft for years. These reactions, which occur between two chemicals (usually a fuel and an oxidizer), are so intense that they can generate propulsion when properly directed. A common example of hypergolic propulsion involves mixing the fuel hydrazine with the oxidizer nitrogen tetroxide. Figure 1 shows A close-up view of the new carbon material, which boasts the highest surface area ever recorded.
At Cornell University, postdoctoral researcher Nikolaos Chalmpes was exploring a novel application of hypergolic reactions. Instead of using them for propulsion, he was leveraging the immense forces generated by chemical combinations to create new materials.
Inspired by the potential to enhance the porosity of carbon, which would increase its surface area and improve its ability to store energy and capture carbon dioxide, Cornell professor Emmanuel Giannelis joined Chalmpes in developing a new study.
“I was trying to understand how to harness and control these unexplored reactions to synthesize various carbon nanostructures, and after adjusting different parameters, I discovered that we might be able to achieve ultrahigh porosity,” said Chalmpes, the study’s lead author. “Until then, these reactions had only been used in rocket and aircraft systems, and deep space probes for propulsion and hydraulic power.”
Working alongside a team of scientists, Chalmpes and Giannelis succeeded in their efforts. They created carbon with a remarkable surface area of 4,800 square meters per gram, which is roughly equivalent to the area of a football field compressed into a teaspoon. "To the best of our knowledge, this area value is the highest reported in the literature," the researchers note in their study, published in the journal ACS Nano.
Five Rings of Carbon
The material's success lies in the fact that the hypergolic reaction produces carbon tubes with a high concentration of molecular rings made up of five carbon atoms, rather than the typical six. This alters the angles of the bonds at the molecular level, enhancing the stability of the tubes.
During the reaction, the tubes self-assembled along a template designed by the researchers, giving the structure its form. The resulting structure was then coated in potassium hydroxide, which removes less stable components, leaving behind thousands of microscopic pores.
“When you conduct this rapid reaction, it creates a perfect scenario where the system can't relax to its lowest energy state, as it normally would,” explained Giannelis. “Due to the speed of hypergolic reactions, you can capture the material in a metastable configuration that wouldn't be achievable through the slow heating of a typical reaction.”
Carbon Capture in Just Two Minutes
After creating the material, the researchers tested its ability to capture carbon dioxide from the atmosphere. In just two minutes, it managed to sequester 99% of its total capacity, a performance nearly double that of current activated carbon products. Additionally, it demonstrated four times the energy storage capability of commercially available activated carbons, with a volumetric energy density of 60 watt-hours per liter.
“This approach provides an alternative strategy for designing and synthesizing carbon-based materials that can serve as sorbents, catalyst supports, and active materials for supercapacitors, especially in applications where space efficiency is critical,” said Chalmpes. “Moreover, the unique conditions of hypergolic reactions open another avenue for designing and synthesizing electrocatalysts with enhanced properties.”
Source:Cornell Chronicle
Cite this article:
Janani R (2024), A Reaction Inspired by Rocket Technology Produces Carbon with An Unprecedented Surface Area, AnaTechMaz, pp. 80