Scientists Solve a Long-Standing Mystery in Chemistry
Computational Chemistry Comes to the Rescue
Similar to traditional palladium catalysis, the reactivity of photoexcited palladium catalysts is highly influenced by the type of phosphine ligand employed. With this in mind, the team proposed that they could pinpoint a suitable phosphine ligand capable of triggering reactivity with alkyl ketones.

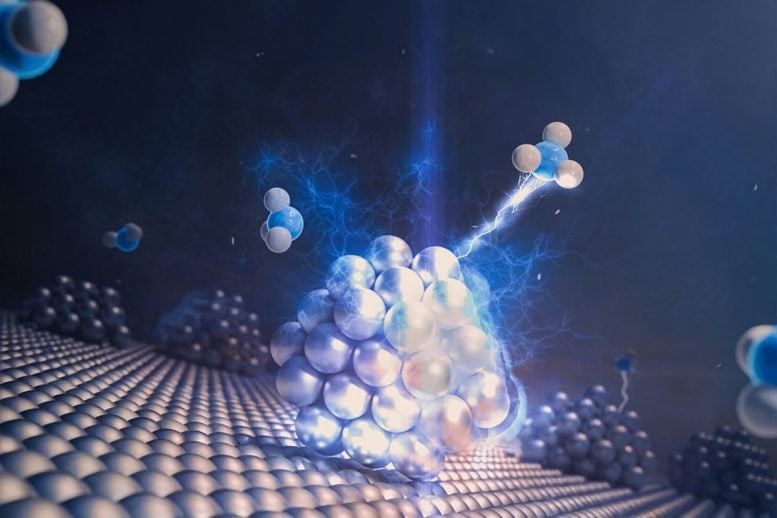
Figure 1. Decades-Old Chemistry Puzzle Finally Solved.
However, given the existence of thousands of known phosphine ligands, experimentally determining the ideal candidate for an untested reaction would be challenging, time-intensive, and environmentally taxing due to the generation of chemical waste. Figure 1. Decades-Old Chemistry Puzzle Finally Solved.
The researchers overcame these challenges by leveraging computational chemistry to efficiently identify optimal ligands with minimal experimental effort. They used the Virtual Ligand-Assisted Screening (VLAS) method, developed by Associate Professor Wataru Matsuoka and Professor Satoshi Maeda from WPI-ICReDD. This approach generated a heat map for 38 different phosphine ligands, predicting which ones were most likely to promote reactivity based on their electronic and steric properties.
Overall, this study not only grants chemists easier access to alkyl ketyl radical reactivity but also demonstrates the power of VLAS in rapidly discovering and optimizing new chemical transformations.
The Mystery Behind the Reaction
For decades, chemists have struggled to understand why certain palladium-catalyzed reactions failed to work with common compounds like alkyl ketones. Despite palladium’s importance in organic synthesis, these limitations hindered progress in creating valuable molecules used in pharmaceuticals, materials, and fine chemicals.
Light Meets Metal — A New Hope
Researchers discovered that photoexcited palladium catalysts—palladium activated by light—could open new reaction pathways. However, not all catalysts behaved as expected. Their reactivity depended heavily on the phosphine ligands attached, and finding the right one proved to be like searching for a needle in a haystack among thousands of known ligands.
Computational Chemistry to the Rescue
Instead of relying on endless lab experiments, the team turned to computational chemistry. They used the Virtual Ligand-Assisted Screening (VLAS) method, developed by scientists at WPI-ICReDD, to simulate how different phosphine ligands might perform. This approach generated a heat map predicting which ligands could drive the desired reactivity based on their electronic and steric properties.
The Perfect Match — Discovering L4
Using the VLAS predictions, researchers tested only three ligands experimentally and found that L4—tris(4-methoxyphenyl) phosphine—was the key. This ligand effectively suppressed back electron transfer (BET), allowing the system to generate ketyl radicals from alkyl ketones and produce chemical reactions with high yields and precision.
A New Era of Chemical Discovery
This breakthrough not only solves a decades-old chemistry challenge but also showcases how AI and computational tools can revolutionize reaction discovery. With methods like VLAS, chemists can design and optimize reactions faster, cleaner, and with less waste—paving the way for a new era of smart, sustainable chemistry.
Source:SciTECHDaily
Cite this article:
Priyadharshini S (2025), Scientists Solve a Long-Standing Mystery in Chemistry, AnaTechMaz, pp. 299