Gold-Based Catalyst Breaks Longstanding Record in Sustainable Chemistry
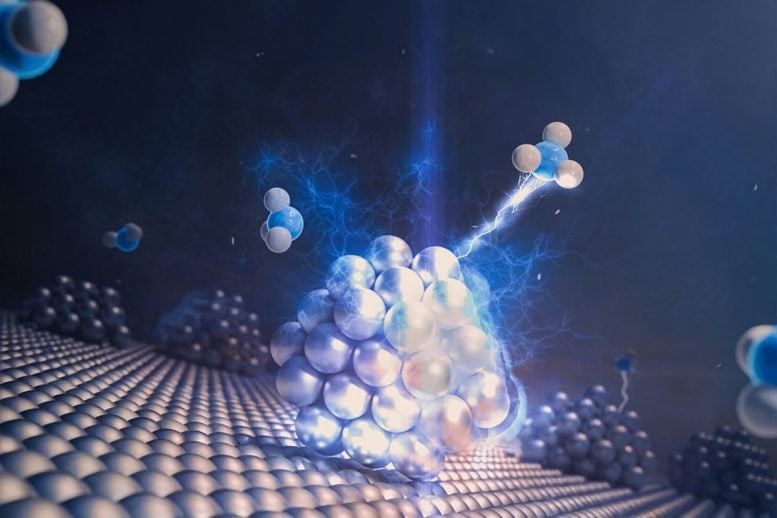
To enhance the efficiency of converting bioethanol into acetaldehyde—a key intermediate for plastics and pharmaceuticals—scientists have developed a new class of gold-based perovskite catalysts. These supports were synthesized via a sol-gel combustion process and subsequently coated with gold nanoparticles. By fine-tuning the manganese-to-copper ratio within the perovskite lattice, the researchers identified an optimal formulation (Au/LaMn₀.₇₅Cu₀.₂₅O₃) that achieved an impressive 95% acetaldehyde yield at 225 °C while maintaining stability for 80 hours.

Figure 1. Record-Breaking Gold Catalyst Redefines Green Chemistry Efficiency.
Catalysts containing higher amounts of copper performed worse, primarily because copper tends to lose its active form during the reaction. The superior performance of the optimized catalyst arises from the synergistic interaction among gold, manganese, and copper ions. Figure 1 shows Record-Breaking Gold Catalyst Redefines Green Chemistry Efficiency.
To uncover the atomic-level mechanism behind this behavior, the team employed advanced computational methods such as density functional theory and microkinetic simulations. Their analysis showed that copper doping in the perovskite structure generates active sites near gold particles, facilitating the activation of oxygen and ethanol molecules. This configuration also lowers the energy barrier for crucial reaction steps, resulting in a more efficient process. The combined experimental and theoretical findings underscore the importance of precisely tuning catalyst composition to maximize performance.
Turning Bioethanol into a Greener Resource
Bioethanol, derived from renewable biomass, can be transformed into acetaldehyde, a vital compound for making plastics, pharmaceuticals, and perfumes. However, achieving high efficiency in this conversion has long been a challenge in green chemistry, limiting its industrial appeal. Scientists have now tackled this by developing a next-generation gold-based catalyst that dramatically improves performance and sustainability.
Designing the Gold–Perovskite Catalyst
The research team created a new class of catalysts built on perovskite materials—a family of compounds known for their versatility in catalysis and energy applications. Using a sol-gel combustion method, they synthesized perovskite supports and coated them with gold nanoparticles. By carefully adjusting the manganese-to-copper ratio, they discovered the ideal composition, Au/LaMn₀.₇₅Cu₀.₂₅O₃, which achieved a remarkable 95% yield of acetaldehyde at 225 °C and remained stable for 80 hours of continuous operation.
Why Copper Content Matters
Not all compositions performed equally well. Catalysts with higher copper content showed poorer results because copper tends to lose its active oxidation state during the reaction. The high-performing catalyst’s success was traced to a cooperative interaction among gold, manganese, and copper ions, which together maintained an ideal balance between activity and stability.
Unraveling the Atomic-Level Mechanism
To understand why this specific formulation worked so efficiently, the team turned to computational chemistry—using density functional theory (DFT) and microkinetic simulations. These analyses revealed that copper doping near gold particles generates highly active sites that promote the activation of both oxygen and ethanol molecules. The optimized structure also lowered the energy barriers for key reaction steps, making the conversion process faster and more energy-efficient.
A Breakthrough for Green Catalysis
The combined experimental and theoretical insights demonstrate how precise atomic-level tuning can dramatically enhance catalyst performance. This gold–perovskite system not only breaks a decade-old efficiency benchmark but also represents a major step forward for sustainable chemical manufacturing. It paves the way for designing next-generation green catalysts capable of transforming renewable resources into valuable industrial chemicals with minimal waste and energy loss.
Source:SciTECHDaily
Cite this article:
Priyadharshini S (2025), Gold-Based Catalyst Breaks Longstanding Record in Sustainable Chemistry, AnaTechMaz, pp. 294