MitraClip: Transcatheter Mitral Valve Repair
MitraClip™ is the world’s first transcatheter mitral valve repair (TMVr) therapy that delivers a minimally invasive treatment option for select patients with primary or secondary mitral regurgitation (MR) who would otherwise go untreated. [1] With more than 17 years of clinical experience and over 100,000 patients treated worldwide, MitraClip™ is a well-established procedure with proven safety and survival, and durable clinical outcomes.
Indication For Use
The MitraClip™ G4 System is indicated for the percutaneous reduction of significant symptomatic mitral regurgitation (MR ≥ 3+) due to primary abnormality of the mitral apparatus [degenerative MR] in patients who have been determined to be at prohibitive risk for mitral valve surgery by a heart team, which includes a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease, [2] and in whom existing comorbidities would not preclude the expected benefit from reduction of the mitral regurgitation. The Figure 1 shows the Mitral Valve Clip.
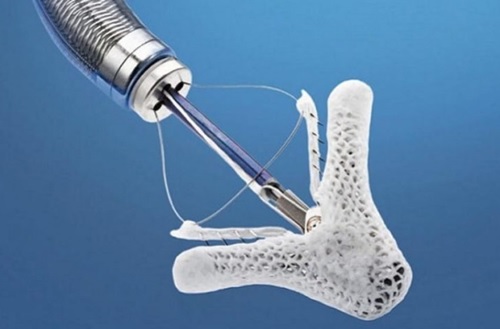
Figure 1: Mitral Valve Clip
The MitraClip™ G4 System, when used with maximally tolerated guideline-directed medical therapy (GDMT), is indicated for the treatment of symptomatic, moderate-to-severe or severe secondary (or functional) mitral regurgitation (MR; MR ≥ Grade III per American Society of Echocardiography criteria) in patients with a left ventricular ejection fraction (LVEF) ≥ 20% and ≤ 50%, and a left ventricular end systolic dimension (LVESD) ≤ 70 mm whose symptoms and MR severity persist despite [3] maximally tolerated GDMT as determined by a multidisciplinary heart team experienced in the evaluation and treatment of heart failure and mitral valve disease.
Contraindications
The MitraClip™ G4 System is contraindicated in patients with the following conditions:
- Patients who cannot tolerate, including allergy or hypersensitivity to, procedural anticoagulation or post procedural anti-platelet regimen
- Patients with known hypersensitivity to clip components (nickel / titanium, cobalt, chromium, polyester), or with contrast sensitivity
- Active endocarditis of the mitral valve
- Rheumatic mitral valve disease
- Evidence of intracardiac, inferior vena cava (IVC) or femoral venous thrombus
Do Not use MitraClip™ outside of the labelled indication.
- The MitraClip™ G4 Implant should be implanted with sterile techniques using fluoroscopy and echocardiography (e.g. transesophageal [TEE] and transthoracic [TTE]) in a facility [4] with on-site cardiac surgery and immediate access to a cardiac operating room.
- Read all instructions carefully. Use universal precautions for biohazards and sharps while handling the MitraClip™ G4 System to avoid user injury. Failure to follow these instructions, warnings and precautions may lead to device damage, user injury or patient injury including:
- MitraClip™ G4 Implant erosion, migration or malposition
- Failure to deliver MitraClip™ G4 Implant to the intended site
- Difficulty or failure to retrieve MitraClip™ G4 system components
- Use caution when treating patients with hemodynamic instability requiring inotropic support or mechanical heart assistance due to the increased risk of mortality in this patient population. The safety and effectiveness of MitraClip™ in these patients has not been evaluated.
- Patients with a rotated heart due to prior cardiac surgery in whom the System is used may have a potential risk of experiencing adverse events such as atrial perforation, cardiac tamponade, tissue damage, and embolism which may be avoided with preoperative evaluation and proper device usage.
- For the Steerable Guide Catheter and Delivery Catheter only:
- The Guide Catheter: the distal 65 cm of the Steerable Guide Catheter with the exception of the distal soft tip, is coated with a hydrophilic coating.
- The Delivery Catheter: coated with a hydrophilic coating for a length of approximately 131 cm.
- Failure to prepare the device as stated in these instructions and failure to handle the device with care could lead to additional intervention or serious adverse event.
- The Clip Delivery System is provided sterile and designed for single use only. Cleaning, re-sterilization and / or reuse may result in infections, malfunction of the device and other serious injury or death.
- Note the product “Use by” date specified on the package.
- Inspect all product prior to use. Do not use if the package is open or damaged, or if product is damaged.
References:
- https://www.cardiovascular.abbott/us/en/hcp/products/structural-heart/transcatheter-valve-solutions/mitraclip.html
- https://www.structuralheartsolutions.com/us/structural-heart-products-solutions/mitral-valve-mitraclip/overview/#isi
- https://mitraclip.com/
- https://www.verywellhealth.com/mitral-valve-clip-5092874
Cite this article:
Vinotha D (2021), MitraClip: Transcatheter Mitral Valve Repair, AnaTechMaz pp. 12