UCLA's Groundbreaking DNA Mapping Uncovers Insights into Human Brain Development
UCLA researchers have created a comprehensive map of brain development, emphasizing DNA modifications in the hippocampus and prefrontal cortex to explore the roots of mental health.
This map is essential for identifying the genetic foundations of neuropsychiatric disorders such as schizophrenia and autism spectrum disorder.
Regulating Genes in Brain Development
A study led by UCLA has uncovered groundbreaking insights into the evolution of gene regulation during human brain development, highlighting the vital importance of chromatin's 3D structure, which consists of DNA and proteins. This research sheds light on how early brain development can impact mental health throughout life.

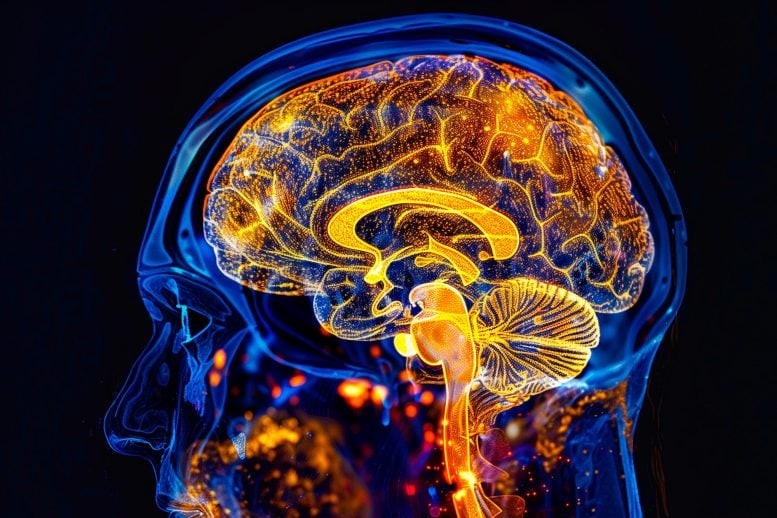
Figure 1. Mapping DNA Modifications for Insights into Brain Development and Neurodevelopmental Disorders
Published in Nature, the study was spearheaded by Dr. Chongyuan Luo at UCLA and Dr. Mercedes Paredes at UC San Francisco, alongside collaborators from the Salk Institute, UC San Diego, and Seoul National University. The team created the first comprehensive map of DNA modification in the hippocampus and prefrontal cortex—crucial brain regions for learning, memory, and emotional regulation, often linked to disorders like autism and schizophrenia. Figure 1 shows Mapping DNA Modifications for Insights into Brain Development and Neurodevelopmental Disorders.
Potential Effects on Neuropsychiatric Disorders
The researchers aim to make their publicly available data resource an invaluable tool for scientists to link genetic variants tied to neuropsychiatric disorders with the relevant genes, cells, and developmental stages most affected. “Neuropsychiatric disorders, even those that manifest in adulthood, often arise from genetic factors that disrupt early brain development,” stated Luo, a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA. “Our map provides a foundational comparison for genetic studies of affected brains, helping to identify when and where molecular changes take place.”
Advancements in Epigenetic Research Methods
To create the map, the research team employed an advanced sequencing method called single nucleus methyl-seq and chromatin conformation capture, or snm3C-seq, developed by Luo and enhanced with assistance from the UCLA Broad Stem Cell Research Center Flow Cytometry Core.
This innovative technique allows researchers to simultaneously examine two epigenetic processes that regulate gene expression at the single-cell level: DNA methylation, a chemical modification of DNA, and chromatin conformation, which refers to the 3D arrangement of tightly folded chromosomes within nuclei. Understanding how these two regulatory mechanisms interact with genes involved in development is essential for uncovering how disruptions in this process can lead to neuropsychiatric disorders.
Connecting Genetic Variants to Gene Regulation
Luo emphasizes that most disease-causing variants are found between genes, making it difficult to identify their regulatory targets. By examining DNA folding within individual cells, researchers can link genetic variants to specific genes, highlighting vulnerable cell types and developmental periods. Understanding genetic risks for conditions like autism spectrum disorder may lead to intervention strategies that alleviate symptoms during critical brain development stages.
Insights from a Comprehensive Developmental Spectrum
The research team examined over 53,000 brain cells from donors ranging from mid-gestation to adulthood, uncovering significant changes in gene regulation during critical developmental stages. By analyzing this wide range of phases, they created a comprehensive picture of the extensive genetic rewiring occurring in human brain development.
One key dynamic period occurs midway through pregnancy when radial glia, neural stem cells that produce billions of neurons, transition to generating glial cells that support and protect neurons. During this time, newly formed neurons mature and establish synaptic connections for communication. This crucial stage has been previously overlooked due to the scarcity of brain tissue from this period.
“Our study addresses the complex interplay between DNA organization and gene expression in developing human brains during often-neglected ages: the third trimester and infancy,” said Paredes, an associate professor of neurology at UCSF. “The connections identified across various cell types could help clarify the challenges in pinpointing significant genetic risk factors for neurodevelopmental and neuropsychiatric conditions.”
Implications for Brain Development Models
The findings also enhance stem cell-based models, like brain organoids, used for studying brain development and diseases. The new map provides a benchmark for researchers to ensure these models accurately reflect human brain development. "Growing a healthy human brain is a tremendous feat," says co-author Dr. Joseph Ecker, a professor at the Salk Institute and Howard Hughes Medical Institute investigator. "Our study creates a vital database that captures key epigenetic changes during brain development, helping us understand where and when failures occur that can lead to neurodevelopmental disorders like autism."
Source: SciTechDaily
Cite this article:
Janani R (2024), UCLA's Groundbreaking DNA Mapping Uncovers Insights into Human Brain Development, AnaTechMaz,pp. 264