Microbiome Therapeutics Manufacturing: Successful Combinations of Extremophiles and Additives Identified
Microbes used for health, agriculture, or other applications must be resilient to extreme conditions and the manufacturing processes involved in tablet production for long-term storage. MIT researchers have now developed a method to enhance the hardiness of these microbes.
Their approach combines bacteria with food and drug additives deemed “generally regarded as safe” by the FDA. The researchers identified formulations that stabilize various types of microbes, including yeast, gram-negative, and gram-positive bacteria, demonstrating that these formulations can endure high temperatures, radiation, and industrial processing that typically harm unprotected microbes. In an even more extreme test, some of the microbes recently returned from the International Space Station, coordinated by Space Center Houston's Manager of Science and Research, Phyllis Friello. The researchers are now analyzing how well these microbes withstood the conditions of space.
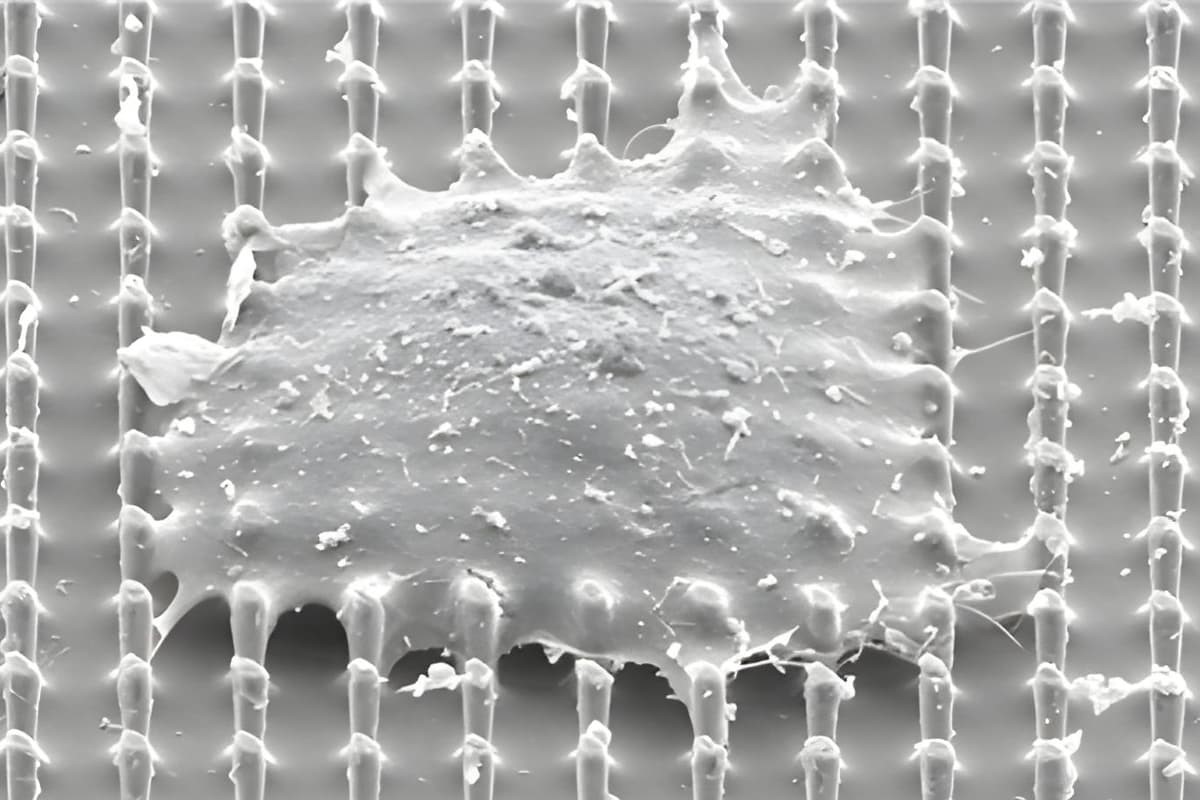
Figure 1. Microbiome Manufacturing: Winning Extremophile and Additive Combinations Found
“What this project is about is stabilizing organisms for extreme conditions,” said Giovanni Traverso, PhD, an associate professor of mechanical engineering at MIT and a gastroenterologist at Brigham and Women’s Hospital. “We’re considering a broad range of applications, whether it’s space missions, human health, or agricultural uses.” Figure 1 shows Microbiome Manufacturing: Winning Extremophile and Additive Combinations Found.
Senior author Traverso, along with lead author Miguel Jimenez, PhD, a former MIT research scientist now an assistant professor of biomedical engineering at Boston University, and their colleagues reported their findings in Nature Materials in a paper titled “Synthetic Extremophiles via Species-Specific Formulations Improve Microbial Therapeutics.” The team concluded that “… these synthetic extremophiles could transform our ability to disseminate bioactive organisms across various applications, from global shelves to agricultural fields and space exploration shuttles.”
Microorganisms traditionally used for food and pharmaceuticals are now being investigated as medicines and agricultural supplements, the authors noted. “Microorganisms have been crucial to human technological advancement and remain essential across various fields, from food production (like baked goods) to biologics manufacturing (such as synthetic insulin).” For these applications, “microbial cells are only kept alive during the manufacturing process and are then destroyed, deactivated, or removed from the final product.”
In contrast, the team explained, “the pharmaceutical, agricultural, and space health sectors are now focused on developing live microorganisms as the final product to treat diseases, enhance crop production, and enable on-demand bioproduction. Maintaining high cell viability throughout the entire product lifecycle is critical for these new microbial technologies.” They suggested that an ideal solution would be dry microbial formulations that are easy to package, ship, and use.
About six years ago, funded by NASA’s Translational Research Institute for Space Health (TRISH), Traverso’s lab initiated research on improving the resilience of beneficial bacteria like probiotics and microbial therapeutics. As an initial step, the researchers evaluated 13 commercially available probiotics and discovered discrepancies in their live bacteria content compared to label claims. “… when we assessed viable cell counts (colony-forming units, CFUs) across various probiotics … we found that only 7 out of 13 products contained viable cell counts equal to or higher than advertised, with an average (geometric) viability of approximately 21% of the promised amount,” they reported.
“What we observed was that, perhaps not surprisingly, there exists a disparity, and it can be significant,” explained Traverso. “So the next question became, given this finding, what actions could we take to address the issue?” In their subsequent experiments, the researchers focused on three bacteria and one yeast. Escherichia coli Nissle 1917 is a probiotic, Ensifer meliloti is a nitrogen-fixing bacterium supporting plant growth in soil, Lactobacillus plantarum is used for fermenting food products, and Saccharomyces boulardii serves as a probiotic yeast.
MIT researchers developed a method to create resilient microbes for medical and agricultural applications by mixing them with about 100 FDA-approved ingredients. This approach identified effective combinations, notably using E. coli Nissle 1917, which showed over 10% survival after six months at 37°C when combined with melibiose, caffeine, or yeast extract. This formulation outperformed commercial products significantly and withstood high radiation levels.
Their formulation was compatible with pharmaceutical processes and maintained microbial function post-exposure to harsh conditions, demonstrating efficacy against pathogens and in nitrogen-fixing. Extremophile microbes were even sent to the International Space Station for stress testing. The team concluded that their high-throughput pipeline could enhance the application of microorganisms in extreme environments and for space exploration. Future work may involve genetic approaches to further improve stability and dosage design.
Source: genengnews
Cite this article:
Janani R (2024), Microbiome Therapeutics Manufacturing: Successful Combinations of Extremophiles and Additives Identified, AnaTechMaz, pp. 259