The Development of Personalized Medicine
Personalized medicine has a fairly broad definition, but, essentially, we're talking about using genetic or other biomarker information to make treatment decisions about patients. These could include decisions about who should get certain kinds of therapies or specific doses of a given therapy, or who should be monitored more carefully because they're predisposed to a particular safety issue. The terms genetics, [1] pharmacogenetics, personalized medicine, and pharmacogenomics have been used interchangeably to mean the study of genetic variations and their influence on the way people respond to medications.
CDER has been developing infrastructure programs and review capacity to be on the leading edge of personalized medicine initiatives. Most recently, we have been working with other FDA centers to make sure that we develop informative and pragmatic guidance and policies on personalized medicine in a timely way.
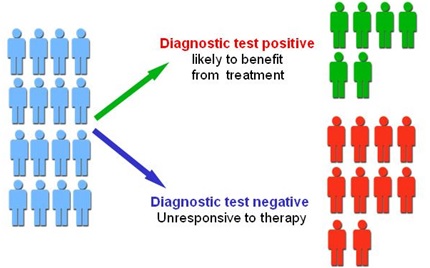
Figure1.Personalized Medicine.
Examples of personalized medicine
- Using methods such as tumor marker testing to treat cancer
- Recommending changes to your sleep habits to improve daytime fatigue
- Genome Sequencing (the process of determining the entire genetic makeup or a specific organism or type of cell)
- Recommendations for changing [2] one’s diet due due to food allergies/sensitivities
- Taking health supplements when your body is deficient to ensure that your body is functioning optimally
- I think it’s working well. The challenge has been that there are a lot of moving parts to personalized medicine.
- Personalized medicine is generally comprised of two elements. One is the drug, biologic, or other therapeutic intervention, and second is the diagnostic test. We have needed to develop not just CDER’s policies, but multi-center policies on personalized medicine that involve the Center for Devices and Radiological Health (CDRH), the Center for Biologics Evaluation and Research, and other FDA centers. In my view, it was pretty challenging to coordinate that interaction from about 2008 to 2011, mainly because the centers were not physically located near each other.
- Now that CDER and CDRH are on the same campus, there's been much closer interaction among the centers as far as joint guidance development and sharing of regulatory review experiences and processes. I see the last year or so as a success and, moving forward, it will be much easier to coordinate now that we are closer and realize more the need to work together to produce some of these important work products.
CDER's involvement in this area
Over the past decade, CDER has been proactive in thinking about personalized medicine in terms of drug development and regulatory decision making. Back in the early 2000s when the human genome project was coming to completion, we had a sense of the amount of genetic variability in the human genome. Senior leaders in the Office of Clinical Pharmacology and the Center articulated a vision for the integration of genomic sciences into regulatory review and drug development. We began publishing on this topic as early as 2001, and have continued over the years.
CDER has been developing infrastructure programs and review capacity to be on the leading edge of personalized medicine initiatives. Most recently, we have been working with other FDA centers to make sure that we develop informative and pragmatic guidance and policies on personalized medicine in a timely way figure1 shown given below.
Personalized medicine, also referred to as precision medicine, is a medical model that separates people into different groups—with medical decisions, practices, [3] interventions and/or products being tailored to the individual patient based on their predicted response or risk of disease.
References:
- en.wikipedia.org/wiki/Personalized medicine
- https://themillsinstitute.com/personalized-medicine-examples
- https://www.fda.gov/drugs/news-events-human-drugs/personalized..
Cite this article:
S. Nandhinidwaraka (2021) The Development of Personalized Medicine, AnaTechMaz, pp. 7