The Potential and Future of Cell Therapy
Cells are the building blocks of life and some cells (stem cells) have the ability to produce other cells through the processes of cell division and cell differentiation. Stem cell research has now progressed dramatically and there are countless studies published every year in scientific journals. Stem cell technology is being used to create new cell lines with edited genes and to regenerate cell-based tissues for biological and medical purposes. This ebook presents a brief snapshot of clinical research in stem cell research and regenerative medicine. The concise reference is intended to be an introduction for biology students to current standards and new technologies in these fields.[1]
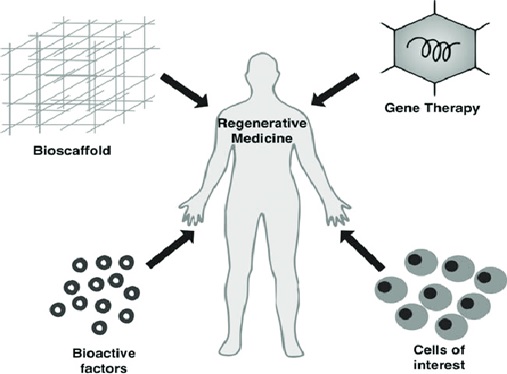
Figure 1. The potential and future of Cell Therapy
Figure 1 shows Engineering cells as a therapeutic is a fundamental leap because cells are the basic functional units of the body and are capable of many sophisticated functions. The relative physical scale alone helps illustrate this point - a cell is ~1000x the size of a biologic. Cells are tiny biological machines that can sense and respond to local milieu, produce material, kill, or defend other cells, seek out targets throughout the body, and regulate their own function over time. Their potential for transformative patient impact across diseases is immense, however little of this opportunity has been realized to date.
Cell therapies today are in their infancy relative to what they could become due in part to two major constraints:
- the difficulty in engineering cell functions; and
- the barrier presented by manufacturing cell therapies at scale.
These constraints mean there are few therapeutic strategies that can be implemented and those that exist often have:
- Tolerability issues and require harsh preconditioning regimens, both of which regularly necessitate hospitalization of the patient
- Limited scope of impact because they are only applicable to certain diseases and only as a last-resort intervention
- Manufacturing concerns that require time, cost, and complexity that limits broad applicability
These limitations are largely why CAR, TCR, TIL, and NK cell therapies are still striving to demonstrate broad applicability for patients. An example of this challenge is the trajectory of cell therapies compared to checkpoint inhibitors. Although CAR-Ts and PD-1/PD-L1 inhibitors both demonstrated clinical proof of concept around the same time (early 2010s), to date the checkpoint inhibitors have been able to treat over a million patients compared to current cell therapies which are in the low thousands.[2]
Linical Landscape
The global market for cell therapy is predominantly shared by stem cells and tissue-specific cells (e.g., skin cells, chondrocytes), followed by blood cells. Current approved stem-cell therapies include hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and to a lesser extent limbal stem cells (LSCs). HSC products are predominantly approved for the treatment of blood disorders. MSC therapies are indicated for a broad variety of diseases, including cardiovascular diseases, graft versus host diseases (GvHD), degenerative disorders, and inflammatory bowel diseases. The single LSC product is approved for LSC deficiency. Distinct from stem cell products, terminally differentiated tissue-specific cells are mainly used for regenerative medicine and tissue engineering applications, such as autologous skin cells (i.e., keratinocytes, fibroblasts, and melanocytes) for the treatment of thermal burns, bi-layers of living cellular skin substitute for venous leg ulcers and diabetic foot ulcers, and autologous chondrocyte scaffolds for repair of cartilage defects.[3]
References:
- https://www.eurekaselect.com/node/150797/toward-the-future-the-new-challenges-of-the-cell-therapy-and-potential-of-regenerative-medicine
- https://www.cellandgene.com/doc/the-potential-and-future-of-cell-therapy-0001
- https://aiche.onlinelibrary.wiley.com/doi/10.1002/btm2.10214
Cite this article:
Thanusri swetha J (2021), The Potential and Future of Cell Therapy, AnaTechMaz, pp. 25