Microfluidics
Microfluidics technology has been applied and adopted across many areas of science and technology over the last 20 years, revolutionising the way patients are diagnosed, monitored and treated. Innovation remains very high in sample analysis and detection and progress has been boosted by the many new tools and procedures created in parallel to enable this.[1]
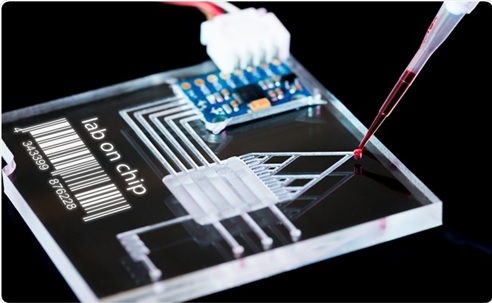
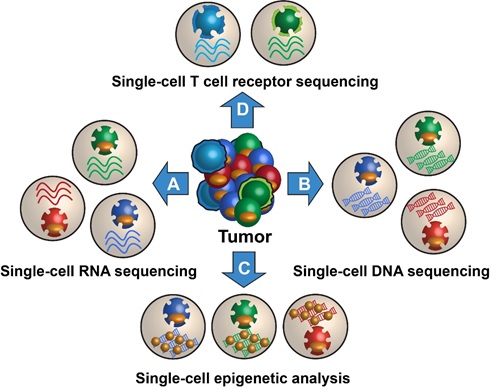
Figure 1. The Future of Microfluidics
Figure 1 shows Microfluidics is the study of systems that can process small quantities of fluids by using tiny channels having dimensions at the microscale – typically tens to hundreds of micrometres.[2]
Advantages of microfluidic systems
- Use of miniscule amounts of samples and reagents in the lab
- Cost reduction due to lesser use of expensive reagents
- High resolution and sensitivity in the detection and separation of molecules
- Reduced footprint of analytical and diagnostic systems compared to huge machines in the lab
- Shorter analysis times and faster results
- Laminar or smooth flow of fluids in tiny channels allows greater flow control
- Greater control of experimental parameters and sample concentration at the micro scale
Applications of microfluidics
- Microfluidic systems are widely used in procedures such as capillary electrophoresis, isoelectric focusing, immunoassays, flow cytometry, sample injection in mass spectrometry, PCR amplification, DNA analysis, separation and manipulation of cells, and cell patterning.
- Research applications of microfluidics are mainly in the study of antibiotic drug-resistant bacteria, nanoparticle transport in blood, and observation of the chemical reaction kinetics.
- Diagnostic uses of microfluidics include cancer and pathogen detection.
- Microfluidic devices are used to measure molecular diffusion coefficients, fluid viscosity, pH, and chemical binding coefficients.
- In the pharmaceutical industry, microfluidic systems have many analytical uses in biopharmaceutical production, e.g., in the monitoring and optimization of protein drugs production and in assays involving human cells.[2]
The future of microfluidics
Microfluidics will be critical for ushering medicine into a new, fast-paced, affordable era. Wearable devices that measure substances in sweat for exercise monitoring and implantable devices that locally deliver cancer drugs to a patient’s tumor are some of the next frontiers of biomedical microfluidics.
Researchers are developing complex, fascinating microfluidic systems called organs-on-a-chip that aim to simulate various aspects of human physiology. In my own lab and other labs across the world, teams are developing tumor-on-a-chip platforms to test cancer drugs more efficiently. These patient avatars will enable scientists to test new treatments in a way that does not entail the cost, suffering and ethical issues associated with testing in animals or in humans. In my lab, we first dissect a tumor biopsy from a cancer patient into thousands of microscopic regular pieces that we keep alive. By virtue of their small size, we can use microfluidics to trap the tiny tumor pieces in multiple wells, one well per drug. These samples retain the appropriate cellular environment of the tumor which will allow us to more accurately predict how a drug will work for a specific person.
Imagine going to the doctor, getting a biopsy extracted, and in less than a week, by using our microfluidic device, the doctor can figure out which drug cocktail works best to remove your tumor. That is still in the future, but what we do know is that the future will be microfluidic.[3]
References:
- https://www.global-engage.com/life-science/what-does-the-future-hold-for-microfluidics/
- https://www.news-medical.net/life-sciences/What-is-Microfluidics.aspx
- https://theconversation.com/microfluidics-the-tiny-beautiful-tech-hidden-all-around-you-160436
Cite this article:
Thanusri swetha J (2021), Microfluidics, AnaTechMaz, pp. 17