Overview of Quantum dots
A quantum dot is a nanometer-sized semiconductor particle traditionally with a core-shell structure. Quantum dots are widely used for their unique optical properties, as they emit light of specific wavelengths if energy is applied to them. These wavelengths of light can be accurately tuned by changing various properties of the particle, including shape, material composition, and size. [2]

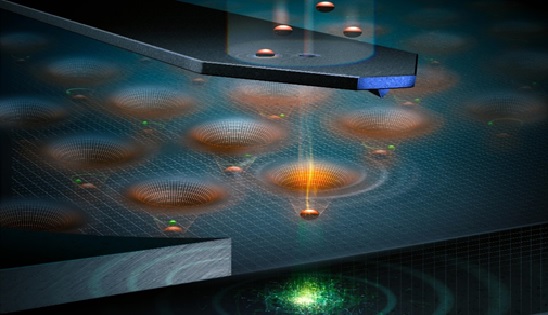
Figure 1. Overview of Quantum dots
Figure 1 shows when energy is applied to an atom, electrons are energised and move to a higher level. When the electron returns to its lower and stable state, this additional energy is emitted as light corresponding to a particular frequency. Quantum dots work in much the same way but a quantum dot crystal acts as one very large atom. The energy source used to stimulate a quantum dot is commonly ultraviolet light. The frequency or colour of light given off is not related to the material used in the quantum dot, but by the size of the quantum dot. [3]
A quantum dot is a small cluster of atoms. For our research in the Surrey University radiation physics team, researchers including me work with quantum dots made from different combinations of elements. If you were to zoom in, you might see cadmium, selenium, indium or zinc atoms, to name just a few.
When we buy our quantum dots for research, they come with a protective shell made from semiconducting materials. The shell helps keep the quantum dot core confined, and provides some stability and protection. During manufacture, scientists can grow a quantum dot and control the diameter of the core and thickness of the shell. [4]
Quantum dots in medicine
Quantum dots enable researchers to study cell processes at the level of a single molecule and may significantly improve the diagnosis and treatment of diseases such as cancers. QDs are either used as active sensor elements in high-resolution cellular imaging, where the fluorescence properties of the quantum dots are changed upon reaction with the analyte, or in passive label probes where selective receptor molecules such as antibodies have been conjugated to the surface of the dots.
Quantum dots could revolutionize medicine. Unfortunately, most of them are toxic. Ironically, the existence of heavy metals in QDs such as cadmium, a well-established human toxicant and carcinogen, poses potential dangers especially for future medical application, where qdots are deliberately injected into the body.
As the use of nanomaterials for biomedical applications is increasing, environmental pollution and toxicity have to be addressed, and the development of a non-toxic and biocompatible nanomaterial is becoming an important issue. [1]
References:
- https://www.nanowerk.com/what_are_quantum_dots.php
- https://avantama.com/what-is-a-quantum-dot/
- https://www.azonano.com/article.aspx?ArticleID=1814
- https://www.britishcouncil.org/voices-magazine/what-quantum-dot
Cite this article:
Thanusri swetha J (2021), Overview of Quantum dots, AnaTechMaz, pp.20