Perovskite Nanocrystals
Perovskite nanocrystals are a class of semiconductor nanocrystals, which exhibit unique characteristics that separate them from traditional quantum dots. Perovskite nanocrystals have an ABX 3 composition where A = cesium, methylammonium (MA), or formamidinium (FA); B = lead or tin; and X = chloride, bromide, or iodide.
Synthetic perovskites have been identified as possible inexpensive base materials for high-efficiency commercial photovoltaics– they showed a conversion efficiency of up to 25.5% reported in 2020 by NREL and can be manufactured using the same thin-film manufacturing techniques as that used for thin film silicon solar cells Methylammonium tin halides and methylammonium lead halides [1] are of interest for use in dye-sensitized solar cells. In July 2016, a team of researchers led by Dr. Alexander Weber-Bargioni demonstrated that perovskite PV cells could reach a theoretical peak efficiency of 31%.
Methylammonium halides are deposited by low-temperature solution methods (typically spin-coating). Other low-temperature (below 100 °C) solution-processed films tend to have considerably smaller diffusion lengths. Stranks et al. described nanostructured cells using a mixed methylammonium lead halide (CH3NH3PbI3−xClx) and demonstrated one amorphous thin-film solar cell with an 11.4% conversion efficiency, and another that reached 15.4% using vacuum evaporation. The film thickness of about 500 to 600 nm implies that the electron and hole diffusion lengths were at least of this order. They measured values of the diffusion length exceeding 1 μm for the mixed perovskite, an order of magnitude greater than the 100 nm for the pure iodide. They also showed that carrier lifetimes in the mixed perovskite are longer than in the pure iodide Liu et al. applied Scanning Photo-current Microscopy to show that the electron diffusion length in mixed halide perovskite along plane is in the order of 10 μm.
Eg ratios are higher than usually observed for similar third-generation cells. With wider bandgap perovskites, VOC up to 1.3 V has been demonstrated.
The technique offers the potential of low cost because of the low temperature solution methods and the absence of rare elements. Cell durability is currently insufficient for commercial use.
Planar heterojunction perovskite solar cells can be manufactured in simplified device architectures (without [2] complex nanostructures) using only vapor deposition. This technique produces 15% solar-to-electrical power conversion as measured under simulated full sunlight above the figure1.
Examples of perovskite:
- Strontium titanate
- Calcium titanate
- Lead titanate
- Bismuth ferrite
- Lanthanum ytterbium oxide
- Silicate perovskite
- Lanthanum manganite
- Yttrium aluminum perovskite (YAP)
- Lutetium aluminum perovskite (LuAP)
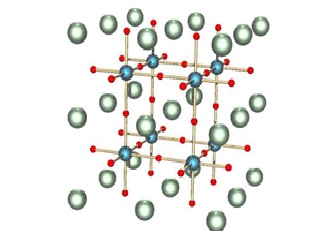
Figure1: Perovskite Nanocrystals
Layered perovskites
Perovskites may be structured in layers, with the ABO3 structure separated by thin sheets of intrusive material. Different forms of intrusions, based on the chemical makeup of the intrusion, are defined as:
- Ruddlesden-Popper phase: the simplest of the phases, the intruding layer occurs between every one (n = 1) or multiple (n > 1) layers of the ABO 3 lattice. Ruddlesden−Popper phases have a similar relationship to perovskites in terms of atomic radii of elements with A typically being large (such as Laor Srwith the B ion being much smaller [3] typically a transition metal (such as Mn, Coor. Recently, hybrid organic-inorganic layered perovskites have been developed, where the structure is constituted of one or more layers of MX64--octahedra, where M is a +2 metal (such as Pb2+ or Sn2+) and X and halide ion (such as F-, Cl-, Br-, I-), separated by layers of organic cations (such as butylammonium- or phenylethylammonium-cation).
References:
- en.wikipedia.org/wiki/Perovskite_nanocrystal
- https://en.wikipedia.org/wiki/Perovskite_nanocrystal
- https://en.wikipedia.org/wiki/Perovskite_(structure)
Cite this article:
Nandhinidwaraka S (2021), Perovskite Nanocrystals, Anatechmaz, pp. 16