Groundbreaking Discovery Unveils Self-Regulation Mechanism of Key Ion Channel
A recent study conducted by researchers at Weill Cornell Medicine offers a precise and detailed illustration of how a common ion channel in mammalian cells regulates itself through a “ball-and-chain” mechanism that blocks the channel.
These findings deepen the understanding of ion channel biology and could aid in developing new drugs targeting these channels to treat conditions such as epilepsy and hypertension.
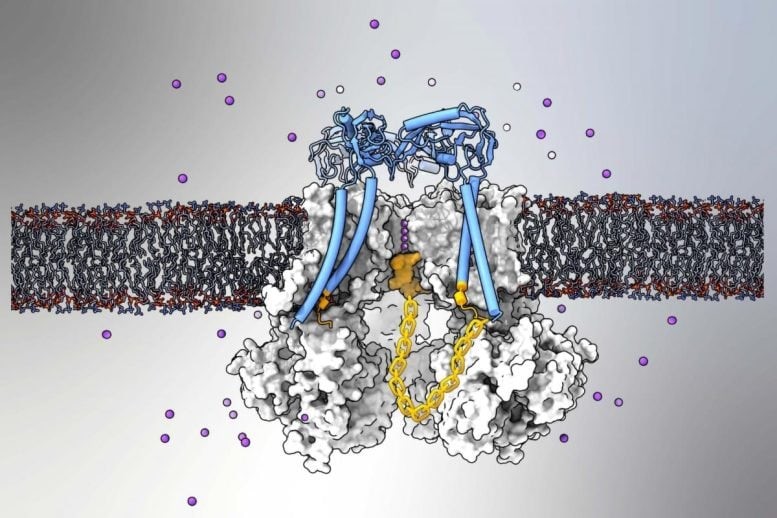
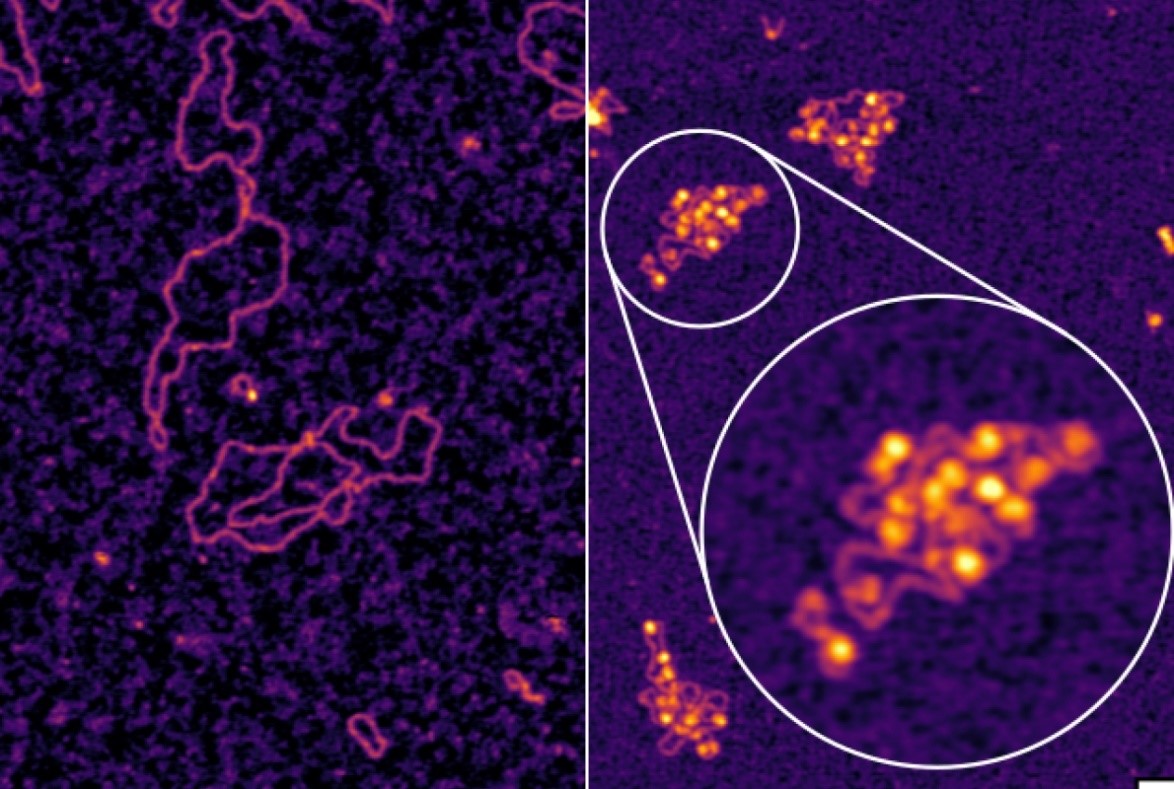
Figure 1. The BK Ion Channel.
Ion channels are protein structures embedded in cell membranes that regulate the movement of charged molecules in and out of cells, playing a vital role in essential biological functions, including brain cell communication. Published on February 19 in Nature Communications, the study focused on the mammalian BK (“big potassium”) channel, which facilitates the outward flow of potassium ions. Figure 1 shows the bk ion channel.
Validating the Ball-and-Chain Mechanism
Using advanced structural imaging and computer modeling techniques, researchers confirmed that BK channels regulate ion flow through a long-theorized ball-and-chain mechanism that blocks the channel.
“These findings offer valuable insights into a fundamental biological process and pave the way for improved methods to modulate ion channel activity for treating human diseases,” said Dr. Crina Nimigean, senior author of the study and professor of physiology and biophysics in anesthesiology at Weill Cornell Medicine [1].
BK channels play a crucial role in regulating the excitability of brain and muscle cells, controlling blood flow in blood vessels, processing auditory signals, and supporting various other bodily functions. Due to this, genetic and other malfunctions in BK channels have been associated with a range of disorders, including epilepsy, movement disorders, hypertension, and hearing-loss syndromes. However, their complexity and fragility have made them challenging to study.
Understanding the Channel’s Self-Regulation
Calcium ions can activate a BK channel by opening its central passage, or “pore,” allowing a substantial outflow of potassium ions from the cell. While previous research had long suggested—but never directly visualized—that BK channels could halt this flow using a ball-like plug attached to a flexible protein subunit, definitive imaging evidence was lacking.
In a widely referenced 2020 study, Dr. Crina Nimigean and her colleagues identified this ball-and-chain structure in MthK, a simpler potassium channel found in bacterial organisms and a distant evolutionary relative of BK channels. In their latest study, the team successfully detected this structure in Slo1, a more complex mammalian BK channel.
To achieve this, the researchers employed low-temperature electron microscopy (cryo-EM), overcoming the challenge of stabilizing the naturally flexible channel structures [2]. Dr. Nimigean’s lab collaborated with Dr. Alessio Accardi, professor of physiology and biophysics at Weill Cornell Medicine, who applied computational modeling techniques to reveal the intricate details of how the protein plug blocks the pore.
“We couldn’t capture a clear cryo-EM image of the pore-binding structure because it binds in multiple conformations,” Dr. Nimigean explained. “Ultimately, with the help of modeling, we discovered that the first three amino acids of the plug are crucial for binding, while the rest form a flexible chain.”
References
- https://www.thenakedscientists.com/articles/science-features/ion-channel-through-keyhole
- https://scitechdaily.com/breakthrough-discovery-reveals-how-key-ion-channel-regulates-itself/
Cite this article:
Keerthana S (2025),Groundbreaking Discovery Unveils Self-Regulation Mechanism of Key Ion Channel,AnaTechMaz,pp. 337