Bacteria Broadcast a Collective Alarm to Counter Threats
Streptococcus pneumoniae bacteria use a spreading communication system to enter a competent state, helping them survive antibiotic stress. Like many communities, bacterial survival relies on sharing strategies to face threats. This species, a leading cause of severe pneumonia and growing antibiotic resistance, gains protection by absorbing useful DNA from its surroundings—a process called competence. However, the way this resistance information spreads through bacterial populations was not well understood.
A team led by molecular microbiologist Patrice Polard at the Centre for Integrative Biology has uncovered how populations of Streptococcus pneumoniae spread competence to resist antibiotics. Published in Nature Communications, their study revealed that competence spreads through bacterial populations like a wave. Understanding this process could provide key insights into how pneumococcus tolerates stress and develops antibiotic resistance, aiding efforts to control its spread.
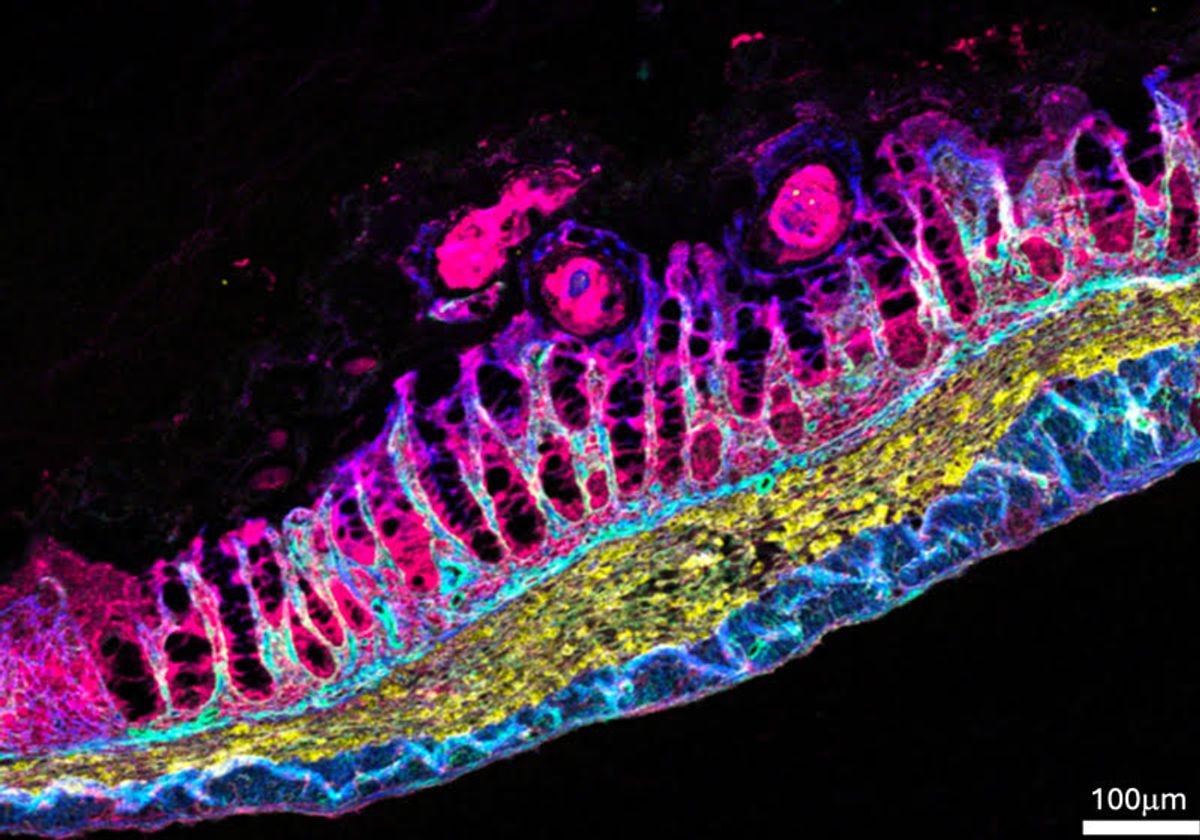
Figure 1. Bacteria Send Population-Wide Alarm
Polard’s team engineered S. pneumoniae strains that fluoresce when cells become competent, enabling real-time tracking of this response. After exposure to a nonlethal dose of streptomycin, only a few cells initially entered competence. However, after about two hours, stress signals amplified across the population, triggering a critical threshold that activated competence in neighboring cells. These competent bacteria then sounded an alarm—likely by releasing Competence Stimulating Peptide (CSP)—to alert and prepare surrounding cells for antibiotic threat. Figure 1 shows Bacteria Send Population-Wide Alarm.
Donald Morrison, a microbiologist at the University of Illinois Chicago not involved in the study, explained, “A subtle stress can be enough to trigger this response and promote a temporary change within a bacterial population.”
Interestingly, not all bacteria that survived the antibiotic exposure reacted the same way. “This diversifies the population,” said Polard. “Some cells become competent while others remain noncompetent.” He added, “This way, some cells survive and others die, but overall, it benefits the entire population.” The researchers believe this strategy helps bacteria cope with environmental stresses by allowing some to acquire useful genes while protecting a portion of the population.
To test whether competence actually improved survival, the team treated bacteria with DNA-damaging antibiotics over time. They found that competent cells survived better and showed greater tolerance to the drugs compared to noncompetent cells.
The team proposed that competence-related antibiotic tolerance could result from slowed growth and metabolic changes. To investigate, they studied the competence-induced protein ComM, which slows cell division. When they genetically deleted the ComM gene, competent cells became less able to tolerate various antibiotics, leading to higher rates of cell death. The researchers believe that by delaying cell division, ComM helps competent cells avoid replicating damaged DNA. Polard’s team is now exploring other competence-activated genes that may also play roles in helping pneumococcus withstand antibiotic stress.
Polard and his team discovered that antibiotics affecting various bacterial functions—such as damaging the cell wall or blocking protein synthesis—also triggered widespread competence throughout the pneumococcus population. Although all antibiotics tested had similar effects on competent cells, the researchers are keen to understand why some antibiotics are more effective against competent cells than others.
Grasping bacterial competence is crucial not only for understanding bacterial evolution and adaptation but also for advancing bacterial genetics and developing new approaches to fight antibiotic resistance. “This simple discovery shows that careless antibiotic use may temporarily induce competence, potentially leading to the evolution of tolerant and resistant cells,” Polard explained.
“Pneumococcus has just 2,000 genes, and we still lack a full understanding of its biology, how it causes disease, and how it spreads,” added Morrison [1]. Investigating competence offers scientists a valuable tool to explore how these genes contribute to the development and spread of antibiotic resistance.
References:
- https://www.the-scientist.com/elevating-wastewater-epidemiology-with-microfluidics-72407
Cite this article:
Janani R (2025), Bacteria Broadcast a Collective Alarm to Counter Threats, AnaTechMaz, pp. 407