Bacterio Rhodopsin Memory (BR)
Bacteriorhodopsin (BR) is one of the simplest and best studied optoelectrical transducers from the microbial (class I) opsins, found in archae, eubacteria, fungi, and algae. It is a protein with seven transmembrane domains that acts like a light-gated active ion pump—it captures photon energy via its covalently bound chromophore, retinal—and moves protons against their electrochemical gradient from the cytoplasm to the extracellular space. Since their discovery,8 the microbial opsins have been viewed as potential components for bioelectronics and a new generation of optical memory because of their ultra-fine spatiotemporal control by light (i.e., their ability to address single molecules by focused light at very fast rates). [1]
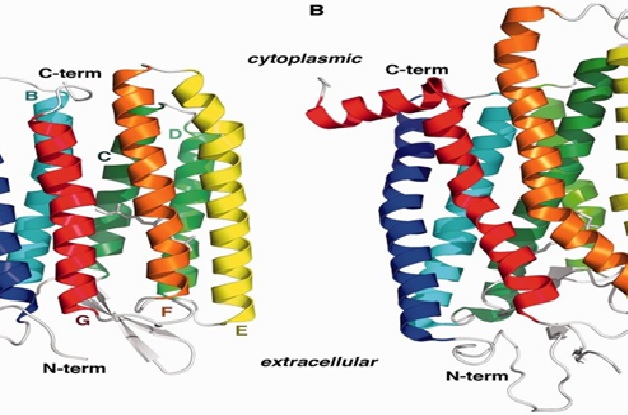
Figure 1. Bacterio Rhodopsin Memory
Figure 1 shows Bacteriorhodopsin is a proton pump found in Archaea, it takes light energy and coverts it into chemical energy, ATP, that can be used by the cell for cellular functions. Bacteriorhodopsin forms chains, which contain retinal molecule within, it is the retinal molecule that absorbs a photon from light, it then changes the confirmation of the nearby Bacteriorhodopsin protein, allowing it to act as a proton pump.
While chlorophyll based ATP generation depends on a protein gradient, like bacteriorhodopsin, but with striking differences, suggesting that phototrophy evolved in bacteria and archaea independently of each other. [2]
Photo Cycle of Bacterio-Rhodopsin
Bacterio-rhodopsin comprises a light absorbing component known as ‘chromophore’ that absorbs light energy and triggers a series of complex internal structural changes to alter the protein’s optical and electrical characteristics. This phenomenon is known as photo cycle.
The initial resting state of the molecule is known as ‘bR’. Green light transforms the initial ‘bR’ state to the intermediate state ‘K’. Next ‘K’ relaxes, forms another intermediate state ‘M’ and then ‘O’ converts to another intermediate state ’P’, which then relaxes to a more stable state ‘Q’. Blue light converts ‘Q’ black to the initial state ‘bR’. Here the idea is to assign any two long-lasting states to the binary values of ‘0’ and ‘1’, to store the required information.
Many of the erstwhile memory devices based on Bacterio-rhodopsin could operate only at extreme cold temperatures of liquid nitrogen, where light-induced switching between ‘bR’ and the intermediate state ‘K’ could be controlled. These devices were much faster than conventional semiconductor-based devices, as these exhibited the speed of a few trillionths of a second. Today, most Bacterio-rhodopsin based devices function even at room temperature, switching between ‘bR’ and another intermediate stable state ‘M’.
If a number of Bacterio-rhodopsin molecules are arranged in a three-dimensional fashion, high-speed, high-density, low-cost memories with vast capacities that can handle large volumes of data can be realized. Such memories offer over 300-fold improvement in storage capacity over their two-dimensional counterparts. [3]
References:
- https://www.sciencedirect.com/topics/medicine-and-dentistry/bacteriorhodopsin
- https://bio.libretexts.org/Bookshelves/Microbiology/Book%3A_Microbiology_(Boundless)/5%3A_Microbial_Metabolism/5.11%3A_Phototrophy/5.11D%3A_Bacteriorhodopsin
- https://www.seminarsonly.com/Bio-Medical-Engineering/Bacterio-Rhodopsin-Memory.php
Cite this article:
Thanusri swetha J (2021) Bacterio Rhodopsin Memory (BR), AnaTechMaz, pp. 31