Innovative Drug Repurposing Unveils Powerful Antibiotic Candidate
A groundbreaking project focused on repurposing existing drugs for potential antibiotic use has identified a highly promising candidate with a unique mechanism for killing drug-resistant bacteria.
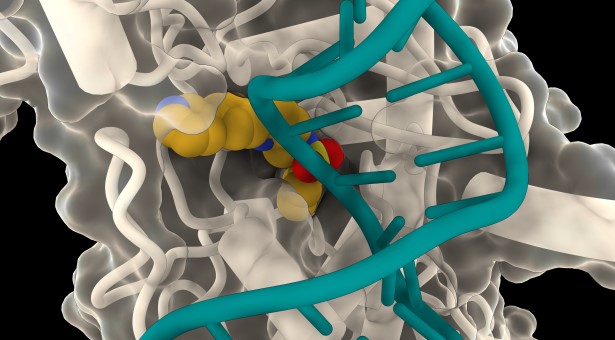
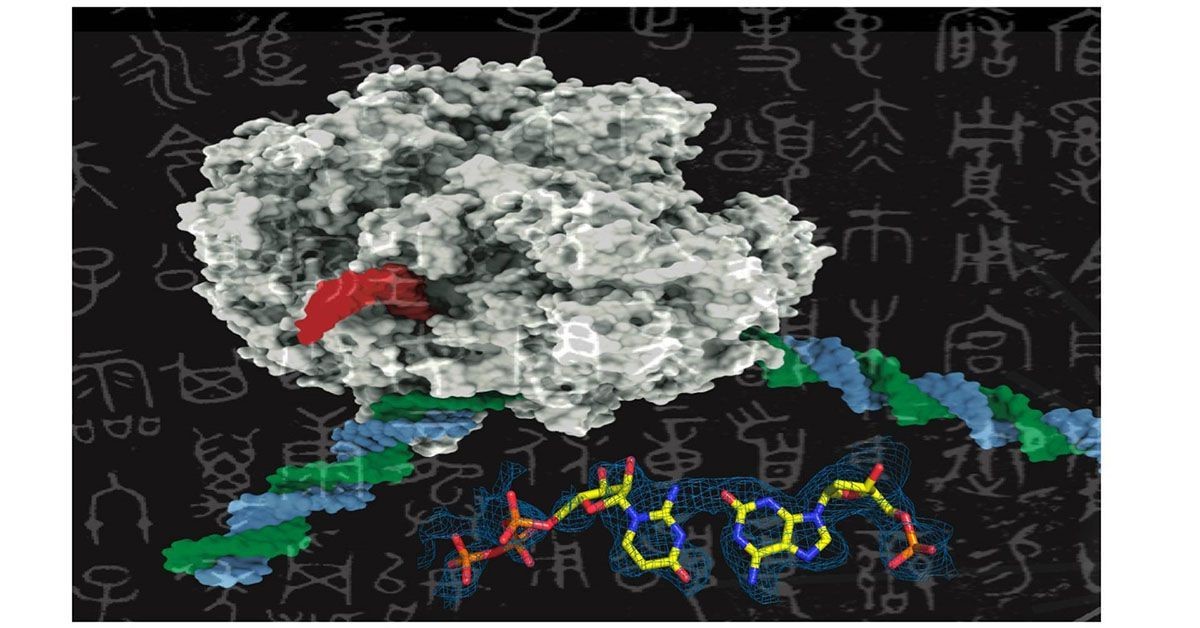
Figure 1. Binding pocket. (Credit: John Innes Centre)
In a collaborative effort between the University of Leiden, Netherlands, and the John Innes Centre, UK, researchers screened a chemical library of 352 small molecules for antimicrobial activity. These molecules, originally used in cancer therapies, had not been tested for their ability to kill harmful bacteria like Escherichia coli. Figure 1 shows binding pocket.
The screen revealed 12 compounds that inhibited bacterial growth, with the most potent being a representative of isoquinoline sulfinamides. Researchers at the University of Leiden chemically optimized this compound, resulting in LEI-800, which exhibited strong antimicrobial activity against E. coli and K. pneumoniae, two gram-negative bacteria often responsible for hospital-acquired infections.
To understand how the compounds killed these harmful bacteria, researchers selected naturally occurring resistant strains. They discovered that all resistant strains had mutations in genes encoding the bacterial enzyme DNA gyrase, indicating this as the compound's target. Gyrase is a bacterial topoisomerase, an enzyme essential for bacterial growth as it breaks and rejoins DNA to remove knots and entanglements.
Gyrase does not occur in humans and is already a target of essential fluoroquinolone antibiotics, whose effectiveness is compromised by growing bacterial resistance. The John Innes team used cryo-EM microscopy to confirm that LEI-800 binds to E. coli gyrase and inhibits it by preventing the enzyme from cutting DNA in a novel way.
The global health emergency requires new antibiotics to combat drug-resistant gram-negative bacteria, and this study paves the way for developing a novel class of molecules targeting these resistant bacteria. Dr. Dmitry Ghilarov from the John Innes Centre expressed excitement about finding a new type of antibiotic through this approach.
Repurposing existing libraries of successful compounds for other uses is valuable as it eliminates the costs associated with synthesizing new libraries. The unique binding pocket of LEI-800 suggests it could offer an additional line of attack when used alongside existing antibiotics that are only partially effective.
Dr. Ghilarov highlighted the potential for more discoveries using this approach, noting the small library screened in this study was designed to target human kinases, yet yielded an entirely novel topoisomerase inhibitor. Given that drug discovery companies typically screen millions of compounds, the potential using this approach is enormous.
Well-known examples of drug repurposing include aspirin, which at low doses acts as a selective anti-blood clot drug, and sildenafil (Viagra), originally developed to control blood pressure but now used for erectile dysfunction.
Dr. Ghilarov emphasized that biology's complexity means a small molecule could bind to more than one target, which is the core of how many medicines have been discovered from nature. Many anti-cancer drugs, such as doxorubicin and bleomycin, are produced by soil bacteria Streptomyces to eliminate their bacterial competitors.
The next steps for this research involve further optimizing LEI-800 and advancing towards clinical trials and commercialisation.
The study, "Discovery of isoquinoline sulfonamides as allosteric gyrase inhibitors with activity against fluoroquinolone-resistant bacteria," appears in Nature Chemistry [1].
Source: John Innes Centre
References:
- https://phys.org/news/2024-06-drugs-approach-enormous-potential-rapid.html
Cite this article:
Hana M (2024), Innovative Drug Repurposing Unveils Powerful Antibiotic Candidate, AnaTechMaz, pp. 250