A Microrna Family Drives the T Cell Response in Cancer
Researchers delved into the impact of microRNAs on T cell memory formation when confronted with conflicting in vitro and in vivo results. In response to a bodily threat, dormant CD8+ T cells transform into cytotoxic agents, undergoing proliferation and generating enzymes ready to eliminate adversaries. After triumphing over the threat, be it infected or cancerous cells, the majority of cytotoxic T cells perish, while a select few evolve into memory T cells, providing ongoing protection against future assaults.
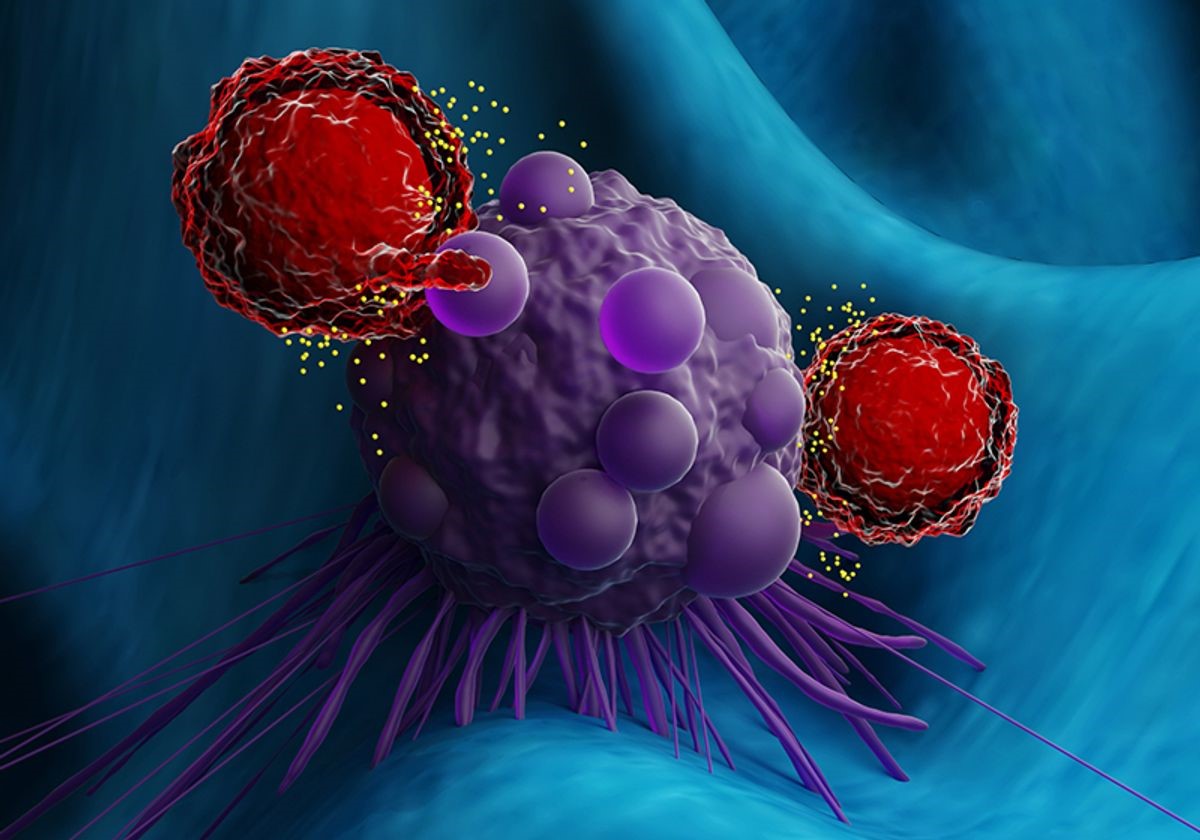
Figure 1.A microRNA Family Drives the T Cell Response in Cancer
Figure 1 Shows A microRNA Family Drives the T Cell Response in Cancer Memory T cells play a crucial role in the adaptive immune response by mounting faster reactions to threats compared to naïve T cells. When encountering a familiar antigen, they quickly transition into effector cytotoxic T cells. The formation of memory T cells is still under investigation, and recent work by immunologists Leonid Pobezinsky and Elena Pobezinskaya from the University of Massachusetts Amherst explores this process in the context of cancer.
Their study, published in Nature Communications, builds on earlier findings from Pobezinsky's group, which identified the significance of let-7, a family of noncoding microRNAs, in the development of cytotoxic T cells. Let-7, known for its role as a tumor suppressor in non-immune cells, directly targets genes involved in cell cycle regulation. While highly expressed in naïve T cells, let-7 is downregulated after the activation of CD8+ T cells. In laboratory experiments, the researchers observed that the absence of let-7 facilitated the proliferation and differentiation of T cells into cytotoxic T cells capable of actively eliminating tumor cells.
In their recent study, the researchers investigated the impact of the let-7 miRNA family on T cell formation in vivo by introducing CD8+ T cells with different let-7 expression levels into mice with melanoma tumors. Surprisingly, the overexpression of let-7 in this context promoted the formation of memory T cells and hindered tumor growth, while cells without these miRNAs were unable to control the tumors. These results contradicted the team's earlier in vitro findings, surprising the researchers.
The researchers propose that the differences observed in the mice can be attributed to the formation of a memory cell pool. To prevent an overly aggressive response that may harm healthy cells, cytotoxic T lymphocytes express inhibitory surface receptors, known as immune checkpoint molecules. Tumors exploit these receptors to evade attack, pushing T cells into a dysfunctional state called exhaustion. In contrast, memory T cells lack certain inhibitory surface receptors, making them invisible to tumors.
According to Pobezinsky, "Mother Nature doesn't want you to inactivate memory cells, which are generated after an immune response because you want to keep them for the rest of your life." When T cells with low let-7 levels were introduced into the melanoma mouse model, the cancer cells likely exploited the inhibitory receptors on cytotoxic T cells, inducing exhaustion. Conversely, T cells expressing let-7 avoided this fate and instead formed memory cells that continued to produce functional cytotoxic T cells in response to cancer antigens. Pobezinsky noted, "We had 70-80 percent tumor-free mice, which is unheard of, especially expressing just one miRNA."
To unravel the cellular mechanisms driving memory formation, the researchers conducted a comparative analysis of the transcriptomes of T cells expressing let-7 and those lacking it. The let-7-expressing cells exhibited significant changes, including the inhibition of pathways crucial for reactive oxygen species (ROS) production, steering CD8+ T cells toward the memory phenotype. To validate this discovery, the scientists treated let-7-deficient T cells with a drug inhibiting a ROS production pathway during early activation. When these treated cells were injected into mice with tumors, they exhibited behavior similar to let-7-expressing T cells—reducing tumor burdens and extending survival.
The research team led by Pobezinsky is currently delving into the mechanisms through which let-7 controls pathways associated with ROS production. Looking ahead, the researchers envision the potential use of let-7 miRNA as an immunotherapy to manage tumor growth, given its role as a tumor suppressor and its influence on memory T cell formation. Ingunn Stromnes, a cancer immunologist at the University of Minnesota who was not involved in the study, expressed optimism about the research, stating, "The authors perform a variety of elegant immunological and transcriptional experimentation that supports that forced overexpression of let-7 may be one strategy to tip the balance in favor of the immune system in the fight against cancer." She also highlighted the importance of understanding how to translate these findings into the human setting, noting the potential feasibility of overexpressing let-7 in non-activated T cells with advancements in gene engineering technologies.
Source:TheScientist
Cite this article:
Janani R (2023),A Microrna Family Drives the T Cell Response in Cancer,AnaTechMaz, pp.151