United Kingdom Approves First-Ever CRISPR Treatment, A Cure for Sickle Cell Disease and Beta Thalassemia
Approval by U.K. regulators for a groundbreaking gene-editing therapy using CRISPR has raised questions about costs, potentially reaching millions of dollars, and concerns about accessibility. The therapy, a world first, targets two inherited blood disorders, including sickle cell disease, which predominantly affects individuals of African ancestry. The treatment involves modifying a patient's blood stem cells in the laboratory and then reintroducing them into the patient.
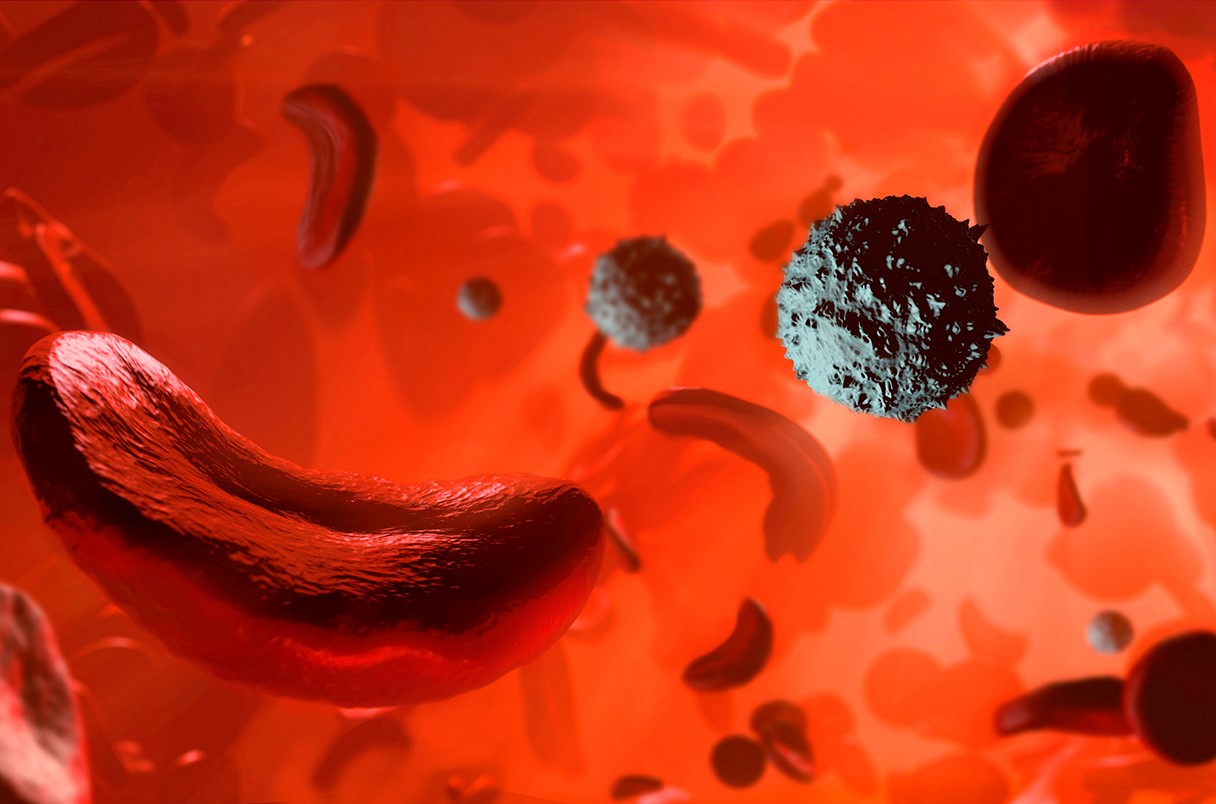
Figure 1.United Kingdom Approves First-Ever CRISPR Treatment, A Cure for Sickle Cell Disease and Beta Thalassemia
Figure 1 Shows United Kingdom approves first-ever CRISPR treatment, a cure for sickle cell disease and beta thalassemia Sickle cell disease is characterized by a defect in the oxygen-carrying protein hemoglobin, leading to the formation of sickle-shaped red blood cells that can obstruct blood vessels, causing intense pain, strokes, and organ damage. The recently approved treatment, developed by Vertex Pharmaceuticals and CRISPR Therapeutics, aims to replace faulty hemoglobin with functional versions encoded by a hemoglobin gene typically active in fetal development. [1] The process involves harvesting a patient's blood stem cells, using CRISPR to disable the genetic switch that normally turns off the fetal hemoglobin gene early in development, and then reintroducing the modified cells into the body. This allows newly produced hemoglobins to assume the oxygen-transporting function.
In clinical trials, the gene-editing treatment for sickle cell disease has shown promising results, with 28 out of 29 patients beginning to produce fetal hemoglobin and experiencing a cessation of severe pain episodes. Similarly, for a related disorder, beta thalassemia, 39 out of 42 patients who received the treatment no longer required blood transfusions to prevent severe anemia. This approach, unlike the currently available cure through bone marrow transplants, which requires a matching donor, involves infusing a patient's own edited cells, eliminating the risk of immune system rejection.
The United Kingdom's Medicines and Healthcare products Regulatory Agency expressed optimism about the approved gene-editing therapy, known as Casgevy, stating that it "has the potential to significantly improve the quality of life for so many.[2] " The therapy, developed by Vertex Pharmaceuticals and CRISPR Therapeutics, has received approval for patients aged 12 and older with sickle cell disease or beta thalassemia.
Anticipation is building for regulators in various countries to follow the United Kingdom's approval of the gene-editing therapy Casgevy. In the United States, an FDA advisory panel recently emphasized the treatment's benefits for sickle cell patients, with FDA approval expected by December 8. European regulators are also reviewing the therapy. Concurrently, bluebird bio's conventional gene therapy for sickle cell disease is anticipated to receive FDA approval this month. Questions about funding from the U.K.’s National Health Service and U.S. insurance companies may arise, given the anticipated high costs of the CRISPR treatment. Additionally, concerns exist about the global accessibility of Casgevy, particularly for individuals in Africa, where most sickle cell disease cases are found and medical facilities capable of administering the complex treatment are limited. The treatment process involves chemotherapy to clear existing blood cells and create space for the edited ones.
References:
- https://www.science.org/content/article/united-kingdom-approves-first-ever-crispr-treatment-cure-sickle-cell-disease-and-beta
- https://www.nytimes.com/2023/11/16/health/casgevy-sickle-cell-crispr.html
Cite this article:
Janani R (2023),United Kingdom Approves First-Ever CRISPR Treatment, A Cure for Sickle Cell Disease and Beta Thalassemia,AnaTechMaz, pp.151