Complexities Of Heart Failure and Ageing in Relation
As the global population ages, there is an increasing demand for precise treatments targeting age-related cardiac health decline. Heart failure, a cardiovascular epidemic on the rise, is primarily influenced by the processes of aging and chronic inflammation. Despite dedicated research to identify the drivers of heart failure and unravel the underlying mechanisms of age-related cardiac decline, effective strategies for prevention and treatment of the root cause have not yet been discovered. The complex nature of age-related heart failure poses a challenge in developing interventions that can effectively address this growing health concern.

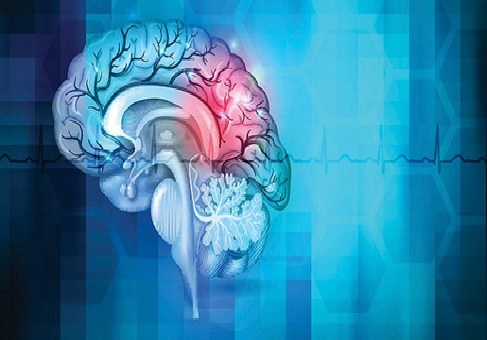
Figure .1 Complexities of Heart Failure and Ageing in Relation
Figure 1 shows Cellular senescence is a state where cells cease to grow and divide upon reaching a certain age. Senescent cells release proteins that contribute to cellular aging and pathological processes. One such protein is insulin-like growth factor-binding protein-7 (IGFBP7), a metabolic protein. Through proteomic analysis, researchers identified IGFBP7 as a potential biomarker for heart failure. In 2017, the American Heart Association recognized the significance of IGFBP7 as a biomarker for heart failure prognosis and diagnosis, based on clinical data. Its involvement in the underlying pathology of heart failure has attracted attention from scientists and clinicians, sparking interest in further understanding its role in this condition.
Peter Liu, the chief scientific officer and vice president of research at the University of Ottawa Heart Institute, was one of the pioneering researchers who identified the role of insulin-like growth factor-binding protein-7 (IGFBP7) in heart failure diagnostics. He addressed the crucial question of whether IGFBP7 is merely a bystander or actively contributes to heart failure. His research team recently published new findings in Nature Cardiovascular Research that shed light on this question.
In their study, Liu's team found that IGFBP7 is overexpressed in the heart and blood of patients with chronic heart failure and in a heart failure mouse model, promoting cardiac cell senescence. They discovered that reducing or neutralizing IGFBP7 opposes the cardiac remodeling process associated with heart failure. The researchers also identified that increased IGFBP7 levels contribute to cardiac remodeling and senescence by suppressing the anti-senescence factor FOXO3a, which hinders DNA repair and reactive oxygen species detoxification.
Liu's team's research builds on previous clinical studies that established a link between IGFBP7 pathways and immune system regulation affecting inflammation, with implications for heart failure and senescence in human patients. Through molecular investigations, they found that genetic removal of IGFBP7 improves heart function and reduces fibrosis, hypertrophy, cell death, and aging signals. These findings demonstrate that IGFBP7 directly contributes to these detrimental consequences associated with heart failure.
We have a cautious amount of optimism that this could be a therapeutic target, according to Liu. Future research on this protein's role in heart failure will focus on therapeutic strategies such antibody-based IGFBP7 blocking or RNA-based therapies that reduce IGFBP7 expression. The good news is that you may actually show a considerable advantage in lowering the severity of heart failure by inhibiting this, by silencing [IGFBP7], according to Gary Lopaschuk, adjunct professor and AHFMR scientist at the University of Alberta who was not involved in the study. "It's a targetable intervention, not just a biomarker," the speaker said.[1]
Liu's research suggests that selectively targeting senescence pathways regulated by IGFBP7 may have therapeutic potential for treating various aging-related conditions beyond heart failure. This is supported by findings indicating that IGFBP7 concentrations can predict both renal and cardiac events in individuals with type 2 diabetes and high cardiovascular risk.
The study highlights the interconnectedness between aging and diabetes, as processes that accelerate aging can lead to diabetes and vice versa. Liu emphasizes the need to consider the synergistic links between the two conditions and the potential benefits of addressing aging and diabetes simultaneously. Identifying molecules like IGFBP7 may provide valuable tools to address the overlapping aspects of aging and diabetes and uncover further therapeutic possibilities.
References:
- https://www.the-scientist.com/news/connecting-the-complexities-of-heart-failure-and-aging-70946
Cite this article:
Janani R (2023), Complexities of Heart Failure and Ageing in Relation, AnaTechMaz,pp.176