The Future Technology Development of Biopharmaceuticals
Biopharmaceuticals are complex medicines made from living cells or organisms, [1] often produced using cutting-edge biotechnological methods.
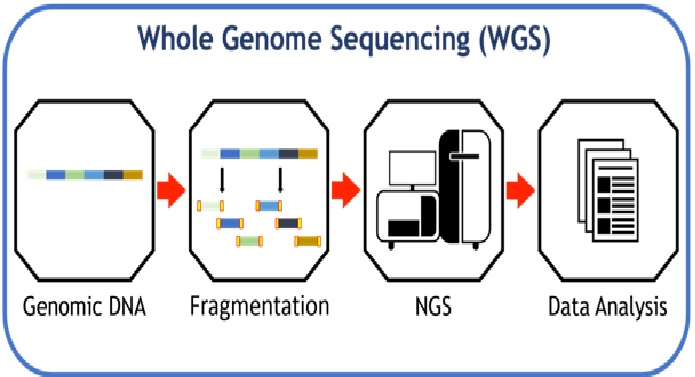
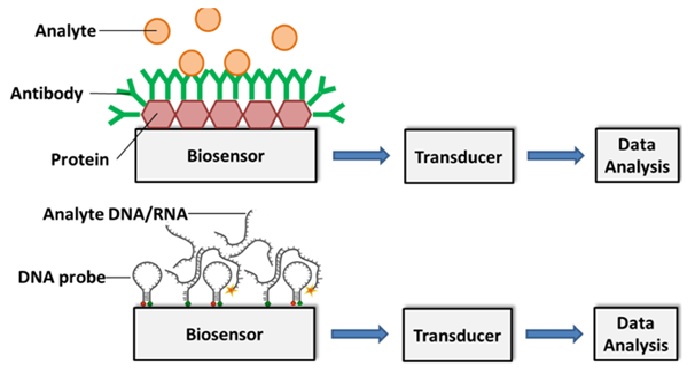
Figure 1: The Biopharmaceutical Process [2]
A biopharmaceutical, also termed a “biologic”, is a medical therapeutic (or drug) that has been synthesized or extracted from a biological source, such as a cell.
The main difference between pharmaceuticals and biopharmaceuticals is that [3] pharmaceuticals have been chemically synthesized whereas biopharmaceuticals have been made using some form of biological source or host.
Biopharmaceuticals are medical drugs produced using biotechnology. They are proteins (including antibodies), nucleic acids (DNA, RNA or antisense oligonucleotides) used for therapeutic or in vivo diagnostic purposes, and are produced by means other than direct extraction from a native (non-engineered) biological source. The technical process is shown in figure 1.
The large majority of biopharmaceutical products are pharmaceuticals that are derived from life forms. Small molecule drugs are not typically regarded as biopharmaceutical in nature by the industry. [4] However members of the press and the business and financial community often extend the definition to include pharmaceuticals not created through biotechnology. That is, the term has become an oft-used buzzword for a variety of different companies producing new, apparently high-tech pharmaceutical products.
When a biopharmaceutical is developed, the company will typically apply for a patent, which is a grant for exclusive manufacturing rights. This is the primary means by which the developer of the drug can recover the investment cost for development of the biopharmaceutical. The patent laws in the United States and Europe differ somewhat on the requirements for a patent, with the European requirements are perceived as more difficult to satisfy. The total number of patents granted for biopharmaceuticals has risen significantly since the 1970s. In 1978 the total patents granted was 30. This had climbed to 15,600 in 1995, and by 2001 there were 34,527 patent applications.
Biopharmaceuticals are among the most sophisticated and elegant achievements of modern science. The huge, complex structures of these drugs don’t just look extraordinary in the 3-D modeling systems used to design them; they also perform their jobs remarkably well, offering high efficacy and few side effects. [5] And there is much more to come: existing treatment archetypes are evolving and becoming more sophisticated all the time, and continuing research is yielding entirely new types of products. Radically new concepts are making it to the market, such as the cell therapy Provenge, which is used to treat cancer, and, somewhat further out, gene therapies, which offer even more amazing promises of regenerative medicine or disease remission.
References:
- https://www.tevapharm.com/product-focus/biopharmaceuticals/what-are-biopharmaceuticals/
- https://www.solvay.com/en/chemical-categories/specialty-polymers/healthcare/biopharma-processing
- https://biopharmaspec.com/biopharmaceuticals/
- https://www.bioprocessonline.com/doc/what-is-biopharmaceutical-0001
- https://www.mckinsey.com/industries/life-sciences/our-insights/rapid-growth-in-biopharma
Cite this article:
Vinotha D (2022), The Future Technology Development of Biopharmaceuticals, AnaTechMaz, pp.197