The Technologies of Direct Air Capture (DAC)
Direct air capture (DAC) technologies extract CO2 directly from the atmosphere. The CO2 can be permanently stored in deep geological formations (thereby achieving negative emissions or carbon removal) or it can be used, [1] for example in food processing or combined with hydrogen to produce synthetic fuels.
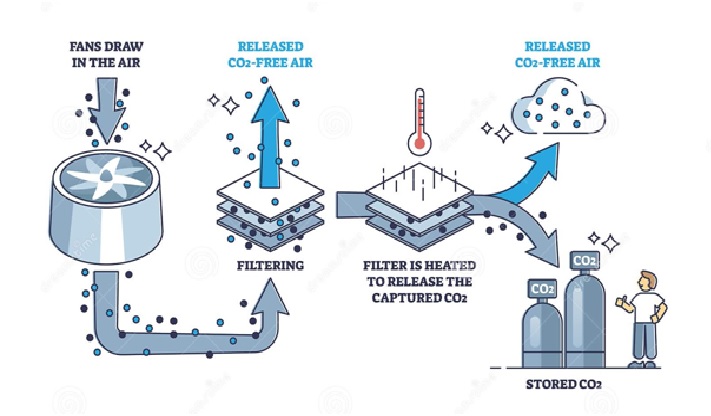
Figure 1: The process of Direct air capture (DAC)[2]
Today, two technology approaches are being used to capture CO2 from the air: liquid and solid DAC. Liquid systems pass air through chemical solutions (e.g. a hydroxide solution), which removes the CO2. The system reintegrates the chemicals back into the process by applying high-temperature heat while returning the rest of the air to the environment.
Solid DAC technology makes use of solid sorbent filters that chemically bind with CO2. When the filters are heated and placed under a vacuum, they release the concentrated CO2, which is then captured for storage or use.
DAC has seen a surge in interest and investment over the past few years and a growing number of companies are entering the space. [3] This is due to the understanding that carbon removal will increasingly be needed to meet national and global climate goals, along with benefits of DAC compared to other carbon removal approaches, which include few practical limits on scaling, relatively little land area use, and siting flexibility.
DAC is currently categorized as “technology readiness level” 6 (on a scale of 1 to 9), meaning it’s still in the large-scale and prototype phase, not yet ready for full commercial deployment. But this also means there’s ample opportunity to improve performance and reduce costs through learning from early iterations of the technology.
The process starts with an air contactor – a large structure modelled off industrial cooling towers. A giant fan pulls air into this structure, where it passes over thin plastic surfaces that have potassium hydroxide solution flowing over them. [4] This non-toxic solution chemically binds with the CO2 molecules, removing them from the air and trapping them in the liquid solution as a carbonate salt.
The CO2 contained in this carbonate solution is then put through a series of chemical processes to increase its concentration, purify and compress it, so it can be delivered in gas form ready for use or storage. This involves separating the salt out from solution into small pellets in a structure called a pellet reactor, which was adapted from water treatment technology. These pellets are then heated in our third step, a calciner, in order to release the CO2 in pure gas form. The calciner is similar to equipment that’s used at very large scale in mining for ore processing. This step also leaves behind processed pellets that are hydrated in a slaker and recycled back into the system to reproduce the original capture chemical.
References:
- https://www.iea.org/reports/direct-air-capture
- https://www.dreamstime.com/direct-air-capture-co-filtering-to-reduce-pollution-outline-diagram-direct-air-capture-co-filtering-to-reduce-pollution-image236891932
- https://www.wri.org/insights/direct-air-capture-resource-considerations-and-costs-carbon-removal
- https://carbonengineering.com/our-technology
Cite this article:
Vinotha D (2022), The Technologies of Direct Air Capture, AnaTechMaz, pp.176