Nature-Inspired Precision: UC Davis Chemists Unlock Chiral Molecule Synthesis
In the intricate world of chemistry, the handedness of organic molecules plays a crucial role, with left- and right-handed versions often having distinct properties. Mimicking nature's efficiency in synthesizing specific chiral molecules has long been a challenge for scientists. However, a breakthrough study from the University of California, Davis, detailed in the January 10 issue of Nature, sheds light on a novel approach using computational modeling and physical experimentation.
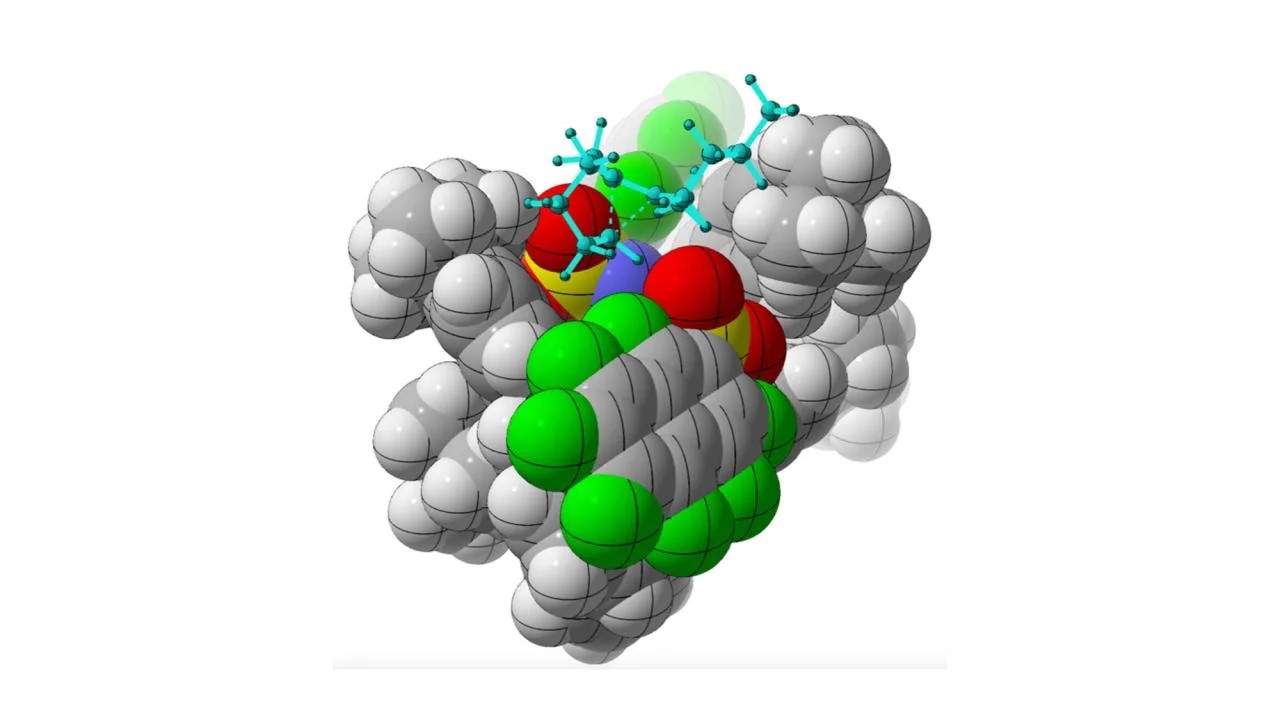
Figure 1. Proposed Method. (Credit: William DeSnoo/UC Davis)
Figure 1 shows chemists at UC Davis are using catalysts (shown in gray spheres) to make organic compounds (blue sticks) with a specific chirality, or “handedness.” Most biological molecules are chiral, including many prescription drugs. The discovery could make it easier to synthesize pharmaceuticals with the correct symmetry.
The Challenge of Chirality
Chirality, akin to the uniqueness of left and right hands, refers to molecules that share the same atomic makeup but are mirror images of each other. The difficulty arises when synthetic chemists aim to produce molecules with specific handedness, crucial for applications like drug development. The inherently greasy nature of these molecules makes achieving selective binding challenging in a laboratory setting.
The Innovative Solution
Professor Dean Tantillo and his team at UC Davis, along with collaborators from the Max Planck Institute in Germany, overcame this challenge by employing a chiral organic acid, imidodiphosphorimidate, as a catalyst. This catalyst facilitated the rearrangement of achiral alkenyl cycloalkanes, resulting in the production of chiral molecules known as cycloalkenes. The breakthrough lies in achieving high selectivity for one chiral form over the other, a significant advancement in the field.
Computational Insights
Utilizing computational methods, Tantillo and his colleagues unraveled the mechanism behind the catalyst's ability to selectively produce one chiral form. The study draws parallels between this synthetic process and the behavior of enzymes in nature that produce hydrocarbon products called terpenes. Understanding and re-engineering these natural processes is a key aspect of Tantillo's broader research.
Applications and Future Prospects
The newly developed method holds promise for harnessing hydrocarbons for various purposes, such as creating precursors for medicines and materials. Tantillo envisions the potential to produce both natural and nonnatural molecules using this innovative approach. Furthermore, with petroleum serving as a rich source of hydrocarbons, catalytically transforming them into molecules with defined chirality could significantly increase their value.
Collaborative Success and Acknowledgments
The collaborative effort involved researchers from the Max Planck Institute in Germany and Hokkaido University in Japan. Financial support for the study came from various sources, including the Max Planck Society, Deutsche Forschungsgemeinschaft, European Research Council, and the U.S. National Science Foundation.
This research marks a significant step forward in the quest to replicate nature's precision in synthesizing chiral molecules. The successful synthesis of specific chiral molecules opens new avenues for diverse applications, potentially revolutionizing the way scientists approach the creation of pharmaceuticals and materials. As the scientific community delves deeper into the possibilities presented by this innovative method, the future holds exciting prospects for advancing the field of synthetic chemistry.
Source: UC Davis
Cite this article:
Hana M (2024), Nature-Inspired Precision: UC Davis Chemists Unlock Chiral Molecule Synthesis, AnaTechmaz, pp. 913

