New Disposable Paper Battery can Activate Adding Water
The battery, devised by Gustav Nyström and colleagues, is made of at least one cell measuring one centimeter squared and consisting of three inks printed onto a rectangular strip of paper.The authors suggest that it could be used to power a wide range of low-power, single-use disposable electronics and minimize their environmental impact.
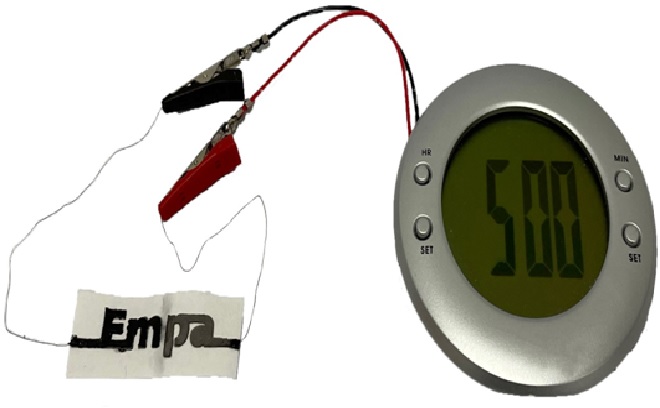
Figure 1: Disposable paper battery can activate adding water.
Figure 1 shows thatSodium chloride salt is dispersed throughout the strip of paper and one of its shorter ends has been dipped in wax. An ink containing graphite flakes, which acts as the positive end of the battery (cathode), is printed onto one of the flat sides of the paper while an ink containing zinc powder, which acts as the negative end of the battery (anode), is printed onto the reverse side of the paper.
Additionally, an ink containing graphite flakes and carbon black is printed on both sides of the paper, on top of the other two inks. This ink connects the positive and negative ends of the battery to two wires, which are located at the wax-dipped end of the paper.[1]
When a small amount of water is added, the salts within the paper dissolve and charged ions are released. The ions activate the battery by dispersing through the paper, resulting in the zinc at the negative end releasing electrons.
Attaching the wires to an electrical device closes the circuit so that electrons can be transferred from the negative end to the positive end, where they are transferred to oxygen in the surrounding air. These reactions generate an electrical current that can power a device.
The researchers demonstrated their creation by combining two cells into a battery that powered an alarm clock with a liquid crystal display. [2]
Analysis of the performance of a one-cell battery revealed that after two drops of water were added, the battery activated within 20 seconds and, when not connected to an energy-consuming device, reached a stable voltage of 1.2 volts.
The voltage of a standard AA alkaline battery is 1.5 volts. After one hour, the one-cell battery’s performance decreased significantly due to the paper drying. However, after two more drops of water were added, it maintained a stable operating voltage of 0.5 volts for more than one additional hour.
The authors suggest that the sustainability of the battery can be further increased by minimizing the amount of zinc used within the ink, which also allows the amount of electricity the battery generates to be precisely controlled. [3]
References:
- https://www.natureasia.com/en/research/highlight/14162
- https://www.imeche.org/news/news-article/paper-battery-powers-single-use-electronics-just-add-water
- https://www.scimex.org/newsfeed/just-add-water-to-activate-this-disposable-paper-battery
Cite this article:
Sri Vasagi K (2022), New Disposable Paper Battery can Activate Adding Water, Anatechmaz, pp. 359

